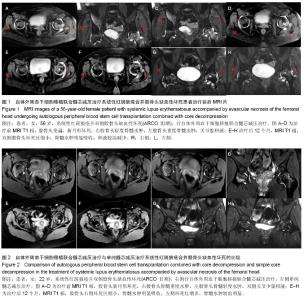
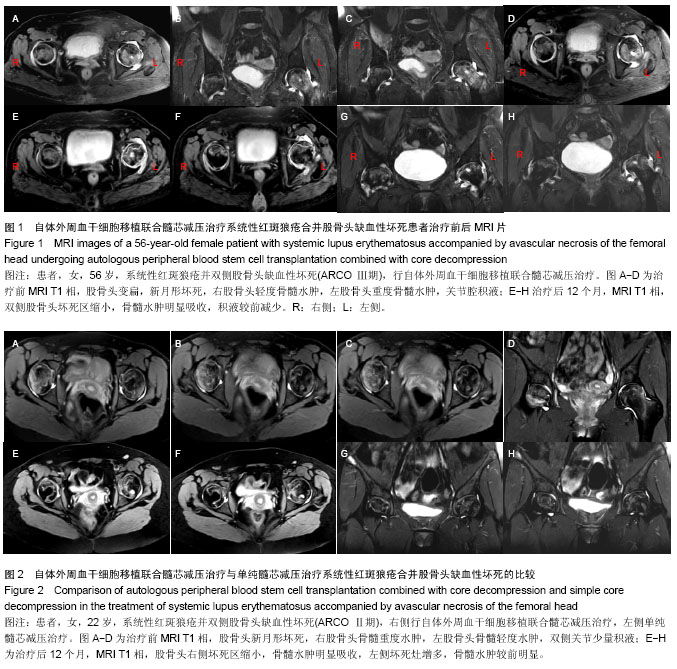
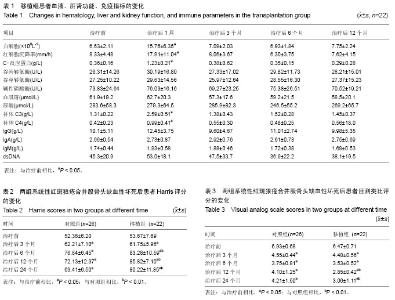
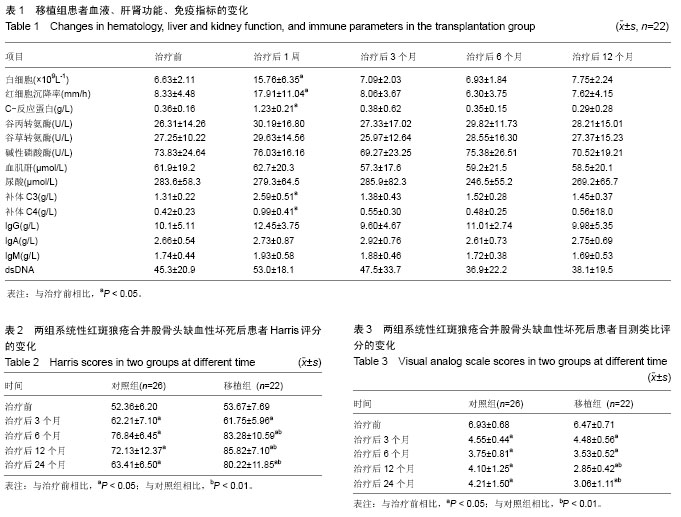
| [1] Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358(9):929-939.
[2] Sfikakis PP, Boletis JN, Tsokos GC. Rituximab anti-B-cell therapy in systemic lupus erythematosus: pointing to the future. Curr Opin Rheumatol. 2005;17(5):550-557.
[3] Cai C, La Cava A. Mimicking self-antigens with synthetic peptides in systemic autoimmune rheumatic diseases. Curr Clin Pharmacol. 2009;4(2):142-147.
[4] Via CS, Handwerger BS. B-cell and T-cell function in systemic lupus erythematosus. Curr Opin Rheumatol. 1993;5(5):570- 574.
[5] Zubler RH, Huang YP, Miescher PA. Mechanisms of physiologic B cell responses and B cell hyperactivity in systemic lupus erythematosus. Springer Semin Immunopathol. 1986;9(2-3):195-218.
[6] Tomietto P, Gremese E, Tolusso B, et al. B cell depletion may lead to normalization of anti-platelet, anti-erythrocyte and antiphospholipid antibodies in systemic lupus erythematosus. Thromb Haemost. 2004;92(5):1150-1153.
[7] Lim SW, Gillis D, Smith W, et al. Rituximab use in systemic lupus erythematosus pneumonitis and a review of current reports. Intern Med J. 2006;36(4):260-262.
[8] Looney RJ, Anolik J, Sanz I. New therapies for systemic lupus erythematosus: cellular targets. Rheum Dis Clin North Am. 2006;32(1):201-215, xi.
[9] Diamanti AP, Rosado MM, Carsetti R, et al. B cells in SLE: different biological drugs for different pathogenic mechanisms. Autoimmun Rev. 2007;7(2):143-148.
[10] 唐福林,金聂,吴敏,等.系统性红斑狼疮并发无菌性骨坏死的危险因素分析[J].中华风湿病学杂志,1999,3(3):166-167.
[11] 张功林,章鸣.带血管骨移植治疗股骨头无菌性坏死进展[J].中国骨伤,2008,21(7):556-557.
[12] 常廷杰,唐康来,陶旭,等.自体血清培养BMSCs移植结合髓芯减压治疗早期股骨头缺血性坏死的初步应用[J].周国修复外科重建杂志,2010,24(6):739-744.
[13] 季卫锋,童培建,郑文标,等.骨髓多能干细胞动脉灌注治疗股骨头坏死的实验研究[J].中国中西医结合杂志,2004,24(11):999- 1002.
[14] 杨晓凤,王红梅,许忆峰,等.干细胞移植改善股骨头坏死缺血状态的实验及临床效应:20只模型兔及188例患者资料分析[J].中国组织工程研究与临床康复,2008,12(8):1558-1562.
[15] Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41-49.
[16] da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204-2213.
[17] 李子荣.股骨头骨坏死的ARCO分期[J].中华外科杂志,1996, 34(3):186-187.
[18] Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
[19] 王天胜,刘永灿,郭利,等.干细胞移植在中晚期股骨头坏死治疗过程中的应用[J].中国组织工程与临床康复,2008,12(3):505-507.
[20] Steinberg ME, Larcom PG, Strafford B, et al. Core decompression with bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2001;(386):71-78.
[21] 沈凌讯,夏菲,余立凯,等.系统性红斑狼疮患者并发无菌性骨坏死的调查分析[J].中华风湿病学杂志,2005,9(1):20-20.
[22] 程少华,崔超,常巍,等.激素性股骨头缺血性坏死细胞凋亡的实验研究[J].中华实用诊断与治疗杂志,2010,24(4):368-370.
[23] 张永乐,王义生,张世清,等.大剂量激素对兔股骨头与股骨近端影响的实验研究[J].中国矫形外科杂志,2012,16(20):1052-1054.
[24] 阮光萍,王金祥,杨建勇,等.系统性红斑狼疮小鼠骨髓间充质干细胞的成骨、成脂分化能力下降[J].中国组织工程研究,2014, 18(1): 1-6.
[25] Yeh CH, Chang JK, Wang YH, et al. Ethanol may suppress Wnt/beta-catenin signaling on human bone marrow stroma cells: a preliminary study. Clin Orthop Relat Res. 2008;466(5): 1047-1053.
[26] 田军,陶天遵,杨林,等.兔股骨头缺血性坏死手术治疗方法的比较[J].临床骨科杂志,2003,6(3):196-199.
[27] Wang GJ, Dughman SS, Reger SI, et al. The effect of core decompression on femoral head blood flow in steroid-induced avascular necrosis of the femoral head. J Bone Joint Surg Am. 1985;67(1):121-124.
[28] 李忠,陈歌,王治,等.髓芯减压复合自体骨髓间充质干细胞移植治疗股骨头缺血坏死[J].中华矫形外科杂志,2012,20(5):411-414.
[29] Ferraresi V, Nuzzo C, Zoccali C, et al. Chordoma: clinical characteristics, management and prognosis of a case series of 25 patients. BMC Cancer. 2010;10:22.
[30] 姜晓华,李忠铁.CD34造血干细胞的研究进展[J].现代医药卫生,2004,20(4):248-249.
[31] Asada T, Kushida T, Umeda M, et al. Prevention of corticosteroid-induced osteonecrosis in rabbits by intra-bone marrow injection of autologous bone marrow cells. Rheumatology (Oxford). 2008;47(5):591-596.
[32] 章乐成,尹宗生.骨髓间充质干细胞及其载体在股骨头坏死治疗中的研究与进展[J].中国组织工程研究,2014,18(3):440-442.
[33] 李瑞琦,张国平,任立中,等.骨髓间充质干细胞治疗股骨头坏死的评价[J].中国组织工程研究,2013,17(35):6327-6331.
[34] 余莲,陈隆天,吴福春,等.自体外周血干细胞移植治疗股骨头缺血性坏死21例[J].中国组织工程研究与临床康复,2008,12(51): 10155-10158.
[35] 王天胜,刘永灿,郭利,等.干细胞移植在中晚期股骨头坏死治疗过程中的应用[J].中国组织工程研究与临床康复.2008,12(3): 505-508.
[36] Gangji V, Toungouz M, Hauzeur JP. Stem cell therapy for osteonecrosis of the femoral head. Expert Opin Biol Ther. 2005;5(4):437-442. |