Chinese Journal of Tissue Engineering Research ›› 2014, Vol. 18 ›› Issue (45): 7348-7352.doi: 10.3969/j.issn.2095-4344.2014.45.024
Previous Articles Next Articles
Stem cell transplantation improves bone mass: research progress and application prospects
Cao De-yu, Li Feng, Qi Bao-chang, Gu Qun, Wang Chun-li, Tao Shu-qing
- Second Department of Orthopedics, Second Affiliated Hospital of Harbin Medical University, Harbin 150001, Heilongjiang Province, China
-
Online:2014-11-05Published:2014-11-05 -
Contact:Tao Shu-qing, Master, Chief physician, Professor, Master’s supervisor, Second Department of Orthopedics, Second Affiliated Hospital of Harbin Medical University, Harbin 150001, Heilongjiang Province, China -
About author:Cao De-yu, Studying for master’s degree, Second Department of Orthopedics, Second Affiliated Hospital of Harbin Medical University, Harbin 150001, Heilongjiang Province, China
CLC Number:
Cite this article
Cao De-yu, Li Feng, Qi Bao-chang, Gu Qun, Wang Chun-li, Tao Shu-qing. Stem cell transplantation improves bone mass: research progress and application prospects [J]. Chinese Journal of Tissue Engineering Research, 2014, 18(45): 7348-7352.
share this article
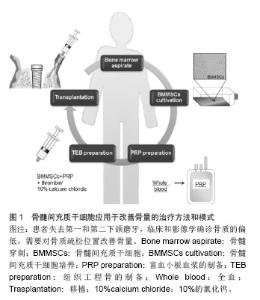
2.1 低骨量的发病率和危害 2.1.1 低骨量的发病率 来自世界卫生组织的报告称,全球50岁以上老人中,1/3的女性和1/5的男性会受到骨质疏松的威胁。随着人口老龄化,中国已居世界骨质疏松症患者数量的首位,在过去的30年间,中国的骨质疏松症患者增加了300%。最新统计结果显示,中国骨质疏松患者达9 000万,其中50岁以上妇女发病率超过50%[5]。 2.1.2 低骨量的危害 低骨量最严重的危害为骨质疏松性骨折,是老年人死亡或残疾的重要因素。北美流行病学调查结果显示,>50岁的白种女性一生中发生常见脆性骨折如髋部骨折的风险为17.5%,临床诊断的椎体骨折为15.6%,前臂远端骨折为16.9%[6]。Xia等[7]研究发现,中国北京地区2002至2006年>50岁女性人群髋部骨折发生率较1990至1992年增高1.5倍,其中>70岁女性人群增高了3.37倍。根据世界卫生组织报道世界范围内由骨质疏松引起的髋关节骨折有170万人,预计到2050年将达到600万[8]。据估计,到2025年仅髋关节骨折会引起每年700 000人死亡,260万人残疾[9-10]。 随着人口的老龄化,骨丢失相关疾病已经逐步影响人类的生活质量。大约有50%的65岁女性在她们的一生中经历过骨质疏松性骨折[11],这些脆弱而低密度骨量的特点,使其易于发生骨折[12]。骨质疏松是一种常见的、与年龄相关的骨疾病,其特点表现为骨量的流失,骨强度的降低及骨组织微观结构的改变。它源于一个不断失衡的过程,即骨形成和骨吸收,易导致骨质脆弱并易于骨折。 骨量在口腔领域也是最重要的一个方面。牙齿的功能不仅对咀嚼功能,而且对演讲、美学、心理满意度和社会福利等功能也是非常重要的[13]。骨内牙齿植入成功的最重要因素则取决于骨量[14],大多数需要牙齿移植的患者为老年人,但他们患有健康问题和其他风险因素,例如佩吉特病、乳腺癌、牙周疾病或骨质疏松症。 这种骨质密度和愈合能力的不断降低可能是由于间充质干细胞的缺陷,即增殖和成骨细胞分化能力的降低[15]。在先前的研究中,Bruder等首次提出系统性管理间充质干细胞自体增殖,可有选择性的治疗临床上富有挑战性的疾病,例如骨质疏松症等。 2.2 发生低骨量的细胞学研究 目前比较公认的低骨量主要发病机制是雌激素缺乏导致破骨细胞增殖分化,破骨细胞功能活跃,同时抑制破骨细胞凋亡,从而使骨吸收速度超过骨形成速度,造成骨质有机物和无机物成比例地减少。另外,雌激素受体(ER)、维生素D受体(VDR)、Ⅰ型胶原和转化生长因子β等基因多态性与骨质疏松关系密切。骨质疏松患者中存在骨生成、骨丢失、骨重建的不平衡,这种不平衡又与成骨细胞、破骨细胞的增殖、分化、凋亡密切关联,而成骨细胞、破骨细胞来源的共同起点是骨髓间充质干细胞。 2.3 骨髓间充质干细胞的特性 骨髓间充质干细胞具备多分化潜能,易于萃取及分离,可以有效的植入低骨量的部位,达到增加骨量的目的。 2.4 干细胞治疗骨量减少的可行性 研究报道,骨髓间充质干细胞能够迁徙、移植到损伤位点,并通过位点的特异性分化过程为其损伤的修复提供一个良好的微环境[11,16]。动物研究表明自体干细胞移植或异体干细胞移植能够增强骨质疏松小鼠的骨量[15-17]。同时也报道了干细胞可通过骨小梁表面直接注入[16]。因此注射骨髓间充质干细胞可以参与受损或病变组织的修复,尤其是在细胞稀少的环境中,需要有大量的特有细胞来修复。 干细胞与生物材料或生长因子的组合可以在几个方面提高细胞疗法的疗效[18]。注射干细胞中富含血小板血浆,它包含诸多生长因子,例如血小板源性生长因子、转化生长因子β、胰岛素样生长因子,这些因子能促进创口愈合和骨组织再生[19]。富含血小板血浆可被凝血酶激活并分泌生物活性因子,对注入的细胞产生一定的影响,这可能会增加治疗效果。再者,注入的细胞能够编织其自然的矩阵结构,此外,骨组织工程支架还可以用来帮助它们确保适当并有差异的增殖[20]。但这样一个细胞疗法可能会影响移植细胞的微环境,通常也会影响移植的干细胞的存活数量[21]。 2.5 干细胞移植改善骨量的尝试 如前所述,低骨量是骨质疏松的一个重要表现,而造成骨量减少的根本原因就是成骨细胞增殖能力减弱甚至丧失,破骨细胞增殖能力及活性增加这种平衡被打破所造成。那么,要从根本上解决骨量减少的问题,就需要从细胞学这个基础进行深入的研究,并尝试应用于临床。 2.5.1 干细胞移植改善骨量的细胞学研究 在动物研究中,自体移植骨髓间充质干细胞到切除卵巢兔股骨,结果显示骨形态结构和机械强度得到明显的改善[15]。自体移植骨髓间充质干细胞注入骨质疏松小鼠股骨内,可提高股骨骨量[16]。马舟涌等[22]将成骨诱导后的脂肪干细胞联合骨形态发生蛋白2/纤维蛋白胶(BMP-2/FG)经皮移植到骨缺损区,结果显示骨缺损区新生骨的数量明显优于对照组,术后8、12周X射线显示骨阻射密度值高于对照组,生物力学检测显示,实验组桡骨标本的四点弯曲断裂载荷均明显高于对照组。唐尤超等[23]将人骨形态发生蛋白2基因修饰的间充质干细胞复合珊瑚羟基磷灰石支架植入骨质疏松大鼠下颌骨缺损部位,4周后可在珊瑚羟基磷灰石边缘发现新生骨质,8周后可见相互连接的板状成熟骨基质,与对照组相比,实验组大鼠新生骨量明显提高。组织测量学和组织分析显示骨髓间充质干细胞治疗组与对照组骨小梁所占百分比与健康大鼠类似。Hsiao等[17]使用从造血细胞污染物中分离骨髓间充质干细胞,由单步塑料贴壁法通过静脉注射的方式移植到骨质疏松小鼠模型内,显示出骨质疏松症状明显改善。 Cho等[24]研究使用反转录病毒对骨髓间充质干细胞过度表达核受体激活剂因子-kappa B(RANK)-Fc和CXC趋化因子受体4(CXCR-4)对去势小鼠静脉移植的影响,他们发现骨髓间充质干细胞过度表达RANK-Fc有效阻止去势小鼠骨量流失同时联合CXCR-4共同控制骨量的流失。另有研究报告使用同种异体脐血单核细胞疗法治疗特发性骨质疏松患者[25],他们在每一个患者的左臂皮下注射细胞,并演示了其对骨密度影响。如上所述,在动物实验中有众多报道称骨髓间充质干细胞可以提高骨量,并能局部长期保留。 2.5.2 一种新的使用间充质干细胞改善骨量的方法 临床研究显示使用以间充质干细胞为依托的组织工程方法对于骨再生的有效性[26-27]。将骨髓间充质干细胞应用于55岁女性患者用于改善骨量,治疗方法和模式如图1所示。"
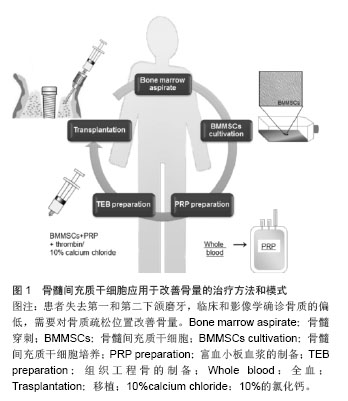
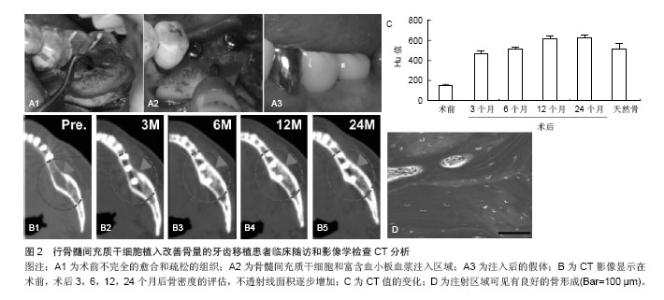
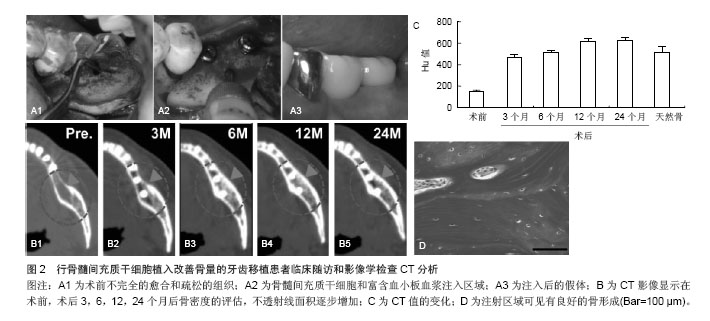
患者失去了第一和第二下颌磨牙,临床和影像学确诊骨质的偏低,需要对骨质疏松位置改善骨量。根据以往的报告方法,在无菌条件下,从患者髂棘抽出骨髓分离培养骨髓间充质干细胞,然后准备200 mL含有枸橼酸抗凝的收集袋收集[26-27],优点在于确保了血小板封存过程的活力,表明血小板计数浓度为257%高于基线值。移植的可用于注射的组织工程骨按照文献的报告方法进行准备[26-27]。简而言之,人类的凝血酶以粉末形式(5 000 U)溶解在10%的氯化钙溶液里。将两个长约13 mm的牙科植入物插入后,通过骨活检针将骨髓间充质干细胞和富含血小板血浆的混合物注射到植入物周围(图2)。唇颊和骨膜按照同样的方式注入。 通常牙齿进行第二次移植手术时,所有的移植物已被完全整合。所植入牙齿的咬合功能已经恢复(图2A)。临床随访和影像学检查CT分析示:骨密度值已经增加,其增加值在3,6,12,24个月分别为471,517,616,626 Hu,高于术前基础水平(149 Hu)(图2B,C)。2年的随访坚持显示没有植入失败的迹象或症状,同时骨质仍然很高。重要的是,随着时间的推移这种变化是稳定的。组织学观察也显示良好的骨形成片状模式和分化良好的骨髓腔(图2D)[26-27]。由此可见,干细胞移植对于骨量的增加,临床效果是显而易见的。"
| [1]程晓光,杨定焯,周琦,等 .中国女性的年龄相关骨密度、骨丢失率、骨质疏松发生率及参考数据库——多中心合作项目[J].中国骨质疏松杂志,2008,14(4):221-228.
[2]中华医学会骨质疏松和骨矿盐疾病分会. 原发性骨质疏松症诊治指南(2011年) [J].中华骨质疏松和骨矿盐疾病杂志,2011,4(1):2-17.
[3]Valverde P.Pharmacotherapies to manage bone loss-associated diseases: a quest for the perfect benefit-to- risk ratio.Curr Med Chem. 2008;15(3):284-304.
[4]Odvina CV, Zerwekh JE, Rao DS,et al.Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90(3):1294-1301.
[5]Lane JM, Riley EH, Wirganowicz PZ.Osteoporosis: diagnosis and treatment.Instr Course Lect. 1997;46:445-458.
[6]Holroyd C, Cooper C, Dennison E.Epidemiology of osteoporosis. Best Pract Res Clin Endocrinol Metab. 2008; 22(5):671-685.
[7]Xia WB, He SL, Xu L,et al.Rapidly increasing rates of hip fracture in Beijing, China.J Bone Miner Res. 2012;27(1): 125-129.
[8]Prevention and management of osteoporosis.World Health Organ Tech Rep Ser. 2003;921:1-164.
[9]Gullberg B, Johnell O, Kanis JA.World-wide projections for hip fracture.Osteoporos Int. 1997;7(5):407-413.
[10]Kanis JA, Oden A, Johnell O,et al.The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int. 2001;12(5):417-427.
[11]Liu Y, Wu J, Zhu Y,et al.Therapeutic application of mesenchymal stem cells in bone and joint diseases.Clin Exp Med. 2014;14(1):13-24.
[12]Tsolaki IN, Madianos PN, Vrotsos JA.Outcomes of dental implants in osteoporotic patients. A literature review.J Prosthodont. 2009;18(4):309-323.
[13]Trulsson M, van der Bilt A, Carlsson GE,et al.From brain to bridge: masticatory function and dental implants.J Oral Rehabil. 2012;39(11):858-877.
[14]Wood MR, Vermilyea SG, Committee on Research in Fixed Prosthodontics of the Academy of Fixed Prosthodontics.A review of selected dental literature on evidence-based treatment planning for dental implants: report of the Committee on Research in Fixed Prosthodontics of the Academy of Fixed Prosthodontics.J Prosthet Dent. 2004; 92(5): 447-462.
[15]Wang Z, Goh J, Das De S,et al.Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model.Tissue Eng. 2006;12(7):1753-1761.
[16]Ocarino Nde M, Boeloni JN, Jorgetti V,et al.Intra-bone marrow injection of mesenchymal stem cells improves the femur bone mass of osteoporotic female rats.Connect Tissue Res. 2010; 51(6):426-433.
[17]Hsiao FS, Cheng CC, Peng SY, et al.Isolation of therapeutically functional mouse bone marrow mesenchymal stem cells within 3 h by an effective single-step plastic-adherent method.Cell Prolif. 2010;43(3):235-248.
[18]Sun HH, Qu TJ, Zhang XH,et al.Designing biomaterials for in situ periodontal tissue regeneration.Biotechnol Prog. 2012; 28(1):3-20.
[19]Marx RE, Carlson ER, Eichstaedt RM,et al.Platelet-rich plasma: Growth factor enhancement for bone grafts.Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638- 646.
[20]Davies JE, Matta R, Mendes VC,et al.Development, characterization and clinical use of a biodegradable composite scaffold for bone engineering in oro-maxillo-facial surgery.Organogenesis. 2010;6(3):161-166.
[21]Mitsiadis TA, Barrandon O, Rochat A,et al.Stem cell niches in mammals.Exp Cell Res. 2007;313(16):3377-3385.
[22]马舟涌,李放,雷伟,等.兔自体脂肪干细胞成骨诱导后经皮注射修复骨缺损[J].中国组织工程研究与临床康复,2008,12(12): 2245-2249.
[23]唐尤超,王远勤,汤炜.基因修饰骨髓间充质干细胞复合珊瑚羟基磷灰石支架材料修复骨质疏松性下颌骨缺损[J].中国组织工程研究与临床康复,2008,12(14):2601 -2605.
[24]Cho SW, Sun HJ, Yang JY,et al.Transplantation of mesenchymal stem cells overexpressing RANK-Fc or CXCR4 prevents bone loss in ovariectomized mice.Mol Ther. 2009;17(11):1979-1987.
[25]Li J, Zhang L, Zhou L,et al.Beneficial effects of non-matched allogeneic cord blood mononuclear cells upon patients with idiopathic osteoporosis.J Transl Med. 2012;10:102.
[26]Yamada Y, Nakamura S, Ito K,et al.Injectable tissue-engineered bone using autogenous bone marrow-derived stromal cells for maxillary sinus augmentation: clinical application report from a 2-6-year follow-up.Tissue Eng Part A. 2008;14(10):1699-1707.
[27]Yamada Y, Nakamura S, Ito K,et al.Injectable bone tissue engineering using expanded mesenchymal stem cells.Stem Cells. 2013;31(3):572-580.
[28]Gangji V, Hauzeur JP, Matos C,et al.Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study.J Bone Joint Surg Am. 2004;86-A(6):1153-1160. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Jiang Hongying, Zhu Liang, Yu Xi, Huang Jing, Xiang Xiaona, Lan Zhengyan, He Hongchen. Effect of platelet-rich plasma on pressure ulcers after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1149-1153. |
| [4] | Tang Hui, Yao Zhihao, Luo Daowen, Peng Shuanglin, Yang Shuanglin, Wang Lang, Xiao Jingang. High fat and high sugar diet combined with streptozotocin to establish a rat model of type 2 diabetic osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1207-1211. |
| [5] | Li Zhongfeng, Chen Minghai, Fan Yinuo, Wei Qiushi, He Wei, Chen Zhenqiu. Mechanism of Yougui Yin for steroid-induced femoral head necrosis based on network pharmacology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1256-1263. |
| [6] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [7] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [8] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [9] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [10] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [11] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [12] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [13] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [14] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [15] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||