Chinese Journal of Tissue Engineering Research ›› 2014, Vol. 18 ›› Issue (3): 348-356.doi: 10.3969/j.issn.2095-4344.2014.03.004
Previous Articles Next Articles
Preliminary fabrication of tissue engineered veins containing valves using bone marrow mesenchymal stem cells and biodegradable scaffolds in vitro
Liu Chi-zhuai 1, 2, Yin Heng-hui1, Lv Wei-ming1, Zeng Chen-guang3, Liu Chang4, Wang Wen-jian1, Quan Da-ping3, Xiang Peng4, Wang Shen-ming1
- 1 Department of Vascular Surgery, the First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China
2 Department of Vascular Surgery, the People’s Hospital of Zhongshan City, Zhongshan 528400, Guangdong Province, China
3 Institute of Polymer Science, School of Chemistry and Chemical Engineering, BME Center, State Key Laboratory of Optoelectronic Materials and Technologies, Sun Yat-sen University, Guangzhou 510275, Guangdong Province, China
4 Center for Stem Cell Biology and Tissue Engineering, Sun Yat-sen University, Key Laboratory for Stem Cells and Tissue Engineering, Ministry of Education, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China
-
Online:2014-01-15Published:2014-01-15 -
Contact:Wang Shen-ming, M.D., Ph.D., Doctoral supervisor, Professor, Department of Vascular Surgery, the First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China -
About author:Liu Chi-zhuai, M.D., Associate chief physician, Department of Vascular Surgery, the First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China; Department of Vascular Surgery, the People’s Hospital of Zhongshan City, Zhongshan 528400, Guangdong Province, China -
Supported by:the National Natural Science Foundation of China, No. 30700804
CLC Number:
Cite this article
Liu Chi-zhuai, Yin Heng-hui, Lv Wei-ming, Zeng Chen-guang, Liu Chang, Wang Wen-jian, Quan Da-ping, Xiang Peng, Wang Shen-ming. Preliminary fabrication of tissue engineered veins containing valves using bone marrow mesenchymal stem cells and biodegradable scaffolds in vitro[J]. Chinese Journal of Tissue Engineering Research, 2014, 18(3): 348-356.
share this article

Characterization of the PLGA scaffold This was prepared using low temperature injection and S-L separation methods. The vein scaffold containing valves was fabricated without any suture materials or additional stent and resembled the natural complex anatomical structure of the human femoral vein. As shown in Figures 2A, B, the scaffold had an inner diameter of 9 mm with a thickness of 0.9 mm. The thickness of the valves was (0.32±0.04) mm. Scanning electron microscopic micrographs demonstrated the presence of regular ladder-like porous structures (Figures 2C, D). The pores exhibited well interconnectivity, and the average pore size and porosity of scaffolds were 10-20 μm and 90% respectively."

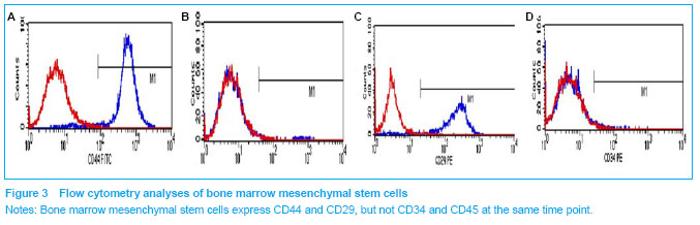
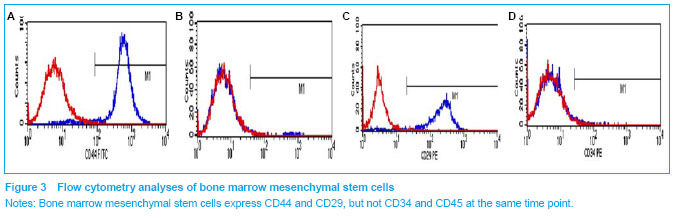
Characterization of bone marrow mesenchymal stem cells Adherent bone marrow mesenchymal stem cells began to exhibit a spindle-shaped morphology by 3 days in culture and expanded rapidly thereafter without contact inhibition. The surface marker expression of the isolated mesenchymal stem cells from the bone marrow was analyzed using flow cytometry (Figure 3). The bone marrow mesenchymal stem cells did not express CD34 and CD45, which are known markers for hematopoietic progenitor cells[10], and differentiated into non-erythrocyte hematopoietic cells respectively. However, these cells were positive for mesenchymal cell markers, CD44 and CD29."
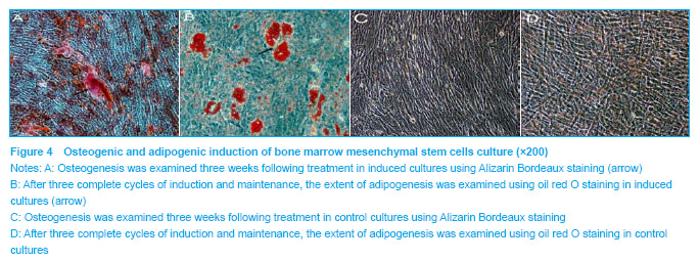
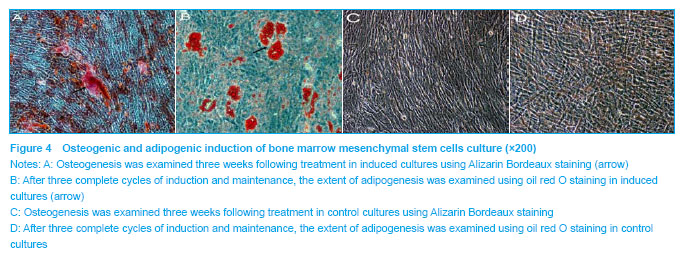
Phenotype of bone marrow mesenchymal stem cells was further confirmed by induction towards the osteogenic and adipogenic lineages. Osteogenesis was examined using Alizarin Bordeaux staining, following three weeks of induction. Alizarin Bordeaux-positive areas appeared red in color in osteogenic cultures (Figure 4A). In contrast, no mineralized bone nodules were found in the control culture (Figure 4C). After three cycles of adipogenic induction and maintenance, lipid-containing cells became very prominent, which were positively stained red by oil red o staining (Figure 4B). In contrast, no oil red o positive cells were found in the cell control culture (Figure 4D). These studies, in combination with the fluorescence-activated cell sorting data, confirmed the mesenchymal stem cell identity of the starting cellular material."
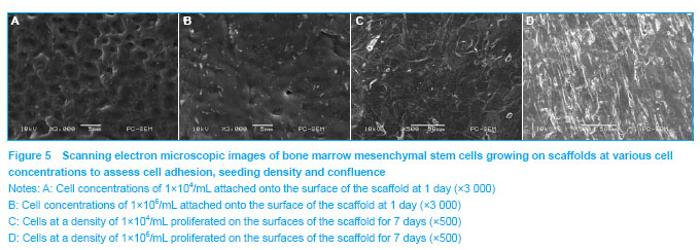
Determination of the optimal cell number for seeding Morphology of the cells and their attachment onto the PLGA scaffolds were evaluated using scanning electron microscope. Scanning electron microscopic assessment showed that the cells attained spherical morphology at the 1st day of culture. Following 3 days in culture, the cells exhibited a spindle-shaped morphology. After a week of culture, the cells had grown and covered almost all the surface of the scaffolds. It was clear that the cells adhered more firmly and formed confluent layer earlier in the group with a cell density of 1×106 cells/mL (Figure 5)."
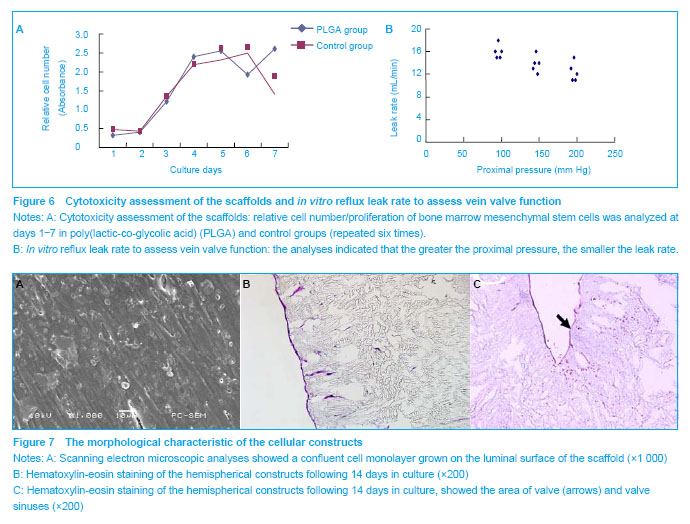
Cell seeding and culture on scaffolds The luminal surface was smooth, glossy and white. The average opening pressure was (3.8±0.7) mm Hg. The valve had an acceptable reflux leakage (Figure 6B). Following 2 weeks of culture, the lumen of the cellular constructs appeared smooth, with flattened cells covering the entire lumen (Figure 7A). Hematoxylin-eosin staining showed that a confluent monolayer of bone marrow mesenchymal stem cells was formed on the luminal surface of the cellular constructs in all of the tissue engineered veins containing valves (Figures 7B, C). These results showed the feasibility of producing a complete tissue engineered vein containing valves from bone marrow mesenchymal stem cells"
| [1]Gohel MS, Barwell JR, Taylor M, et al. Long Term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): randomised controlled trial. BMJ. 2007;335(7610):83. [2]Taheri SA, Schultz RO. Experimental prosthetic vein valve. long-term results. Angiology. 1995;46(4):299-303.[3]Hill R, Schmidt S, Evancho M, et al. Development of a prosthetic venous valve. J Biomed Mater Res.1985; 19(7): 827-832.[4]Pavcnik D, Uchida BT, Timmermans HA, et al. Percutaneous bioprosthetic venous valve: a long-term study in sheep. J Vasc Surg. 2002;35(3):598-602.[5]Teebken OE, Puschmann C, Aper T, et al. Tissue-engineered bioprosthetic venous valve: a long-term study in sheep. Eur J Vasc Endovasc Surg. 2003;25(4): 305-312.[6]Cho SW, Park HJ, Ryu JH, et al. Vascular patches tissue-engineered with autologous bone marrow-derived cells and decellularized tissue matrices. Biomaterials. 2005; 26(14):1915-1924.[7]Zhao Y, Zhang S, Zhou J, et al. The development of a tissue-engineered artery using decellularized scaffold and autologous ovine mesenchymal stem cells. Biomaterials. 2010;31(2):296-307.[8]The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.[9]Gong Z, Niklason LE. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (Hmscs). FASEB J. 2008;22(6):1635-1648.[10]Cho SW, Lim SH, Kim IK, et al. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann Surg. 2005;241(3):506-515.[11]Teebken OE, Puschmann C, Breitenbach I, et al. Preclinical development of tissue-engineered vein valves and venous substitutes using re-endothelialised human vein matrix. Eur J Vasc Endovasc Surg. 2009;37(1):92-102.[12]Kabbani L, Escobar GA, Mansour F, et al. Longevity and outcomes of axillary valve transplantation for severe lower extremity chronic venous insufficiency. Ann Vasc Surg. 2011;25(4):496-501.[13]Akesson H, Risberg B, Björgell O. External support valvuloplasty in the treatment of chronic deep vein incompetence of the legs. Int Angiol. 1999;18(3): 233-238.[14]Maleti O, Perrin M. Reconstructive surgery for deep vein reflux in the lower limbs: techniques, results and indications. Eur J Vasc Endovasc Surg. 2011;41(6):837-848. [15]Pavcnik D, Uchida B, Kaufman J, et al. Percutaneous management of chronic deep venous reflux: review of experimental work and early clinical experience with bioprosthetic valve. Vasc Med. 2008;13(1):75-84. [16]Gale SS, Shuman S, Beebe HG, et al. Percutaneous venous valve bioprosthesis: initial observations. Vasc Endovascular Surg. 2004;38(3):221-224.[17]Wang Z, Zhang Z, Zhang JC, et al. Distribution of bone marrow stem cells in large porous polyester scaffolds. Chin Sci Bull. 2009;54(17):2968-2975.[18]Soletti L, Hong Y, Guan J, et al. A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts. Acta Biomater. 2010;6(1):110-122. [19]Zhang WJ, Liu W, Cui L, et al. Tissue engineering of blood vessel. J Cell Mol Med. 2007;11(5):945-957.[20]Shao HJ, Chen CS, Lee IC, et al. Designing a three-dimensional expanded polytetrafluoroethylene- poly(lactic-co-glycolic acid) scaffold for tissue engineering. Artif Organs. 2009;33(4): 309-317. [21]Cancedda R, Giannoni P, Mastrogiacomo M. A Tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials. 2007;28(29): 4240-4250. [22]Ma Z, Gao C, Gong Y, et al. Paraffin spheres as porogen to fabricate poly(l-lactic acid) scaffolds with improved cytocompatibility for cartilage tissue engineering. J Biomed Mater Res B Appl Biomater. 2003;67(1):610-617.[23]Hu X, Shen H, Yang F, et al. Preparation and cell affinity of microtubular orientation-structured plga(70/30) blood vessel scaffold. Biomaterials. 2008;29(21):3128-3136.[24]Wu L, Zhang J, Jing D, et al. "Wet-State" mechanical properties of three-dimensional polyester porous scaffolds. J Biomed Mater Res A. 2006;76(2):264-271.[25]Xie S, Zhu Q, Wang B, et al. Incorporation of tripolyphosphate nanoparticles into fibrous poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2010;31(19):5100-5109. [26]Leung L, Chan C, Baek S, et al. Comparison of morphology and mechanical properties of PLGA bioscaffolds. Biomed Mater. 2008;3(2):025006. [27]Oh SH, Kang SG, Kim ES, et al. Fabrication and characterization of hydrophilic poly(lactic-co-glycolic acid)/poly(vinyl alcohol) blend cell scaffolds by melt-molding particulate-leaching method. Biomaterials. 2003;24(22):4011-4021.[28]O'Cearbhaill ED, Murphy M, Barry F, Et al. Behavior of human mesenchymal stem cells in fibrin-based vascular tissue engineering constructs. Ann Biomed Eng. 2010; 38(3):649-657. [29]Zhang L, Zhou J, Lu Q, et al. A Novel small-diameter vascular graft: in vivo behavior of biodegradable three-layered tubular scaffolds. Biotechnol Bioeng. 2008;99(4):1007-1015.[30]Hashi CK, Zhu Y, Yang GY, et al. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci U S A. 2007;104(29): 11915-11920. [31]Matsumura G, Miyagawa-Tomita S, Shin'oka T, et al. First Evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003;108(14):1729-1734. [32]O'Cearbhaill ED, Punchard MA, Murphy M, et al. Response of mesenchymal stem cells to the biomechanical environment of the endothelium on a flexible tubular silicone substrate. Biomaterials. 2008;29(11):1610-1619. 42(3):467-475. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [4] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [5] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [6] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [7] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [8] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [9] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [10] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [11] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [12] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [13] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [14] | Kong Desheng, He Jingjing, Feng Baofeng, Guo Ruiyun, Asiamah Ernest Amponsah, Lü Fei, Zhang Shuhan, Zhang Xiaolin, Ma Jun, Cui Huixian. Efficacy of mesenchymal stem cells in the spinal cord injury of large animal models: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1142-1148. |
| [15] | Chen Junyi, Wang Ning, Peng Chengfei, Zhu Lunjing, Duan Jiangtao, Wang Ye, Bei Chaoyong. Decalcified bone matrix and lentivirus-mediated silencing of P75 neurotrophin receptor transfected bone marrow mesenchymal stem cells to construct tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 510-515. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||