Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (23): 5964-5971.doi: 10.12307/2026.349
Previous Articles Next Articles
Association between environmental exposure to endocrine disrupting chemicals and the risk of type 1 diabetes
Wang Ting1, Yang Yang1, Li Yuping2, Yang Lin1
- 1Department of Clinical Medicine, Yangzhou University Medical College, Yangzhou 225009, Jiangsu Province, China; 2Department of Neurosurgery, Northern Jiangsu People’s Hospital Affiliated to Yangzhou University, Yangzhou 225001, Jiangsu Province, China
-
Received:2025-06-27Accepted:2025-08-14Online:2026-08-18Published:2025-12-30 -
Contact:Yang Lin, PhD, Associate professor, Department of Clinical Medicine, Yangzhou University Medical College, Yangzhou 225009, Jiangsu Province, China -
About author:Wang Ting, Department of Clinical Medicine, Yangzhou University Medical College, Yangzhou 225009, Jiangsu Province, China -
Supported by:Key Research and Development Project of the Science and Technology Plan of Yangzhou City, Jiangsu Province, No. YZ2024068 (to LYP); Key Laboratory Project for Big Data Analysis and Knowledge Services, a Jointly Built Science and Technology Innovation Platform between Yangzhou City and Yangzhou University, No. YBK202202 (to LYP)
CLC Number:
Cite this article
Wang Ting, Yang Yang, Li Yuping, Yang Lin. Association between environmental exposure to endocrine disrupting chemicals and the risk of type 1 diabetes[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 5964-5971.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
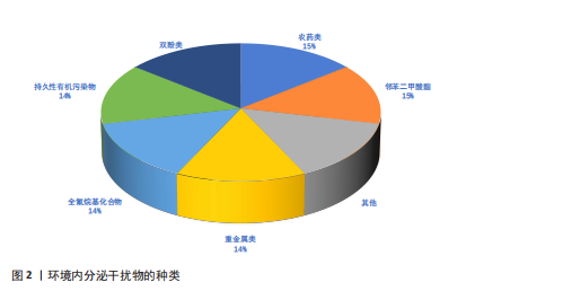
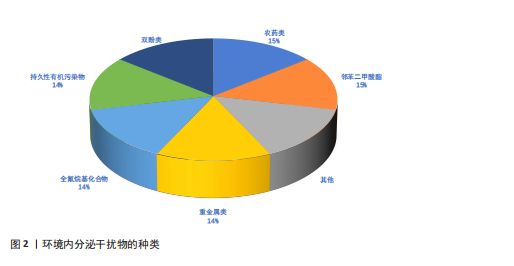
2.1 环境内分泌干扰物的来源与分类 2.1.1 环境内分泌干扰物的环境来源及其暴露途径 环境内分泌干扰物的环境来源与人类活动密切相关,尤其是工业、农业和城市生活领域。工业排放是环境内分泌干扰物进入环境的重要途径之一,生产和使用过程中会有大量化学品释放到空气、水体和土壤中[5]。农业活动中使用的农药和化肥,可通过雨水途径流进入水中,造成水污染。此外,家庭和商业活动中使用的清洁剂、个人护理品和塑料制品等,也可能成为环境内分泌干扰物的来源。这些物质可以通过多种途径进入人体,主要包括食物摄入、皮肤接触和吸入。例如,双酚A和邻苯二甲酸酯等塑化剂常从食品包装材料渗入到食物中,进而被人体摄入[6-7]。研究表明,儿童由于生理和行为特征,在饮食和日常生活中对环境内分泌干扰物的暴露风险更高[8]。因此,了解环境内分泌干扰物的来源和暴露途径对于评估其对人类健康风险至关重要。 2.1.2 常见环境内分泌干扰物的种类 环境内分泌干扰物作为一类在生活环境中普遍存在的化学物质,具有干扰生物体内分泌系统的能力。依据化学结构及来源,环境内分泌干扰物可划分为多种类型,包括重金属、农药、塑化剂等(图2)。铅、汞、镉等重金属元素在工业与农业领域广泛应用,已被确认为重要的环境内分泌干扰物。此类重金属可通过食物链富集,对生物体产生显著的毒性效应,尤其对胚胎发育期及儿童成长阶段构成更大威胁[9]。农药,尤其是有机氯和有机磷类农药,其内分泌干扰活性已获证实,可对生殖系统和发育过程产生不良影响[4]。双酚A和邻苯二甲酸酯等塑化剂广泛应用于生产塑料及各类合成材料,这些物质在生产和使用环节易释放到环境介质中,并通过食品包装材料及日常消费品等途径进入人体,干扰内分泌稳态,进而引发代谢紊乱等一系列健康问题[10-11]。综上,环境内分泌干扰物种类繁多,其对生态环境及人类健康的潜在风险不容忽视。 2.2 环境内分泌干扰物对免疫系统的影响 2.2.1 环境内分泌干扰物对免疫细胞功能的影响 免疫系统是环境内分泌干扰物的关键靶标之一。环境内分泌干扰物对免疫细胞功能的调控效应引起学界高度关注。研究表明,环境内"
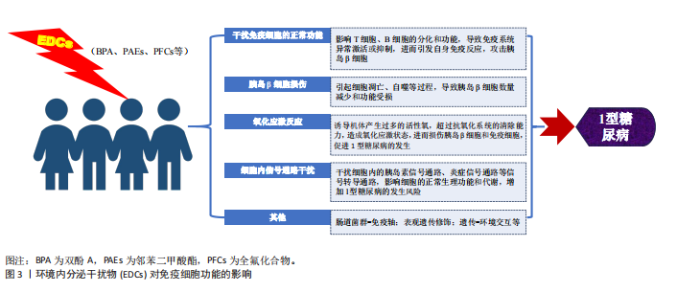
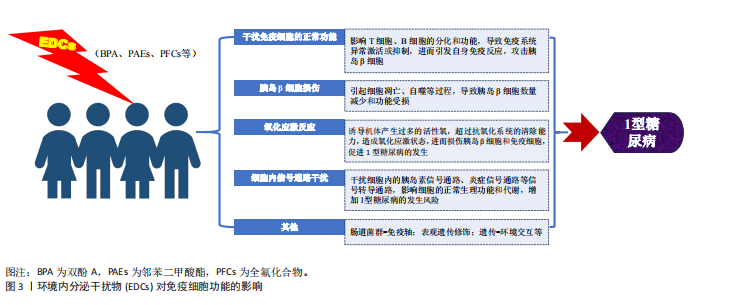
分泌干扰物通过多重机制干扰免疫细胞的功能活性和生存周期。例如,特定环境内分泌干扰物(如邻苯二甲酸酯和双酚A)已被证实可显著影响包括单核细胞、淋巴细胞、树突状细胞及自然杀伤细胞在内的多种免疫细胞的功能[12],其作用机制可能涉及对特定激素受体的激活或拮抗,进而扰动免疫细胞的关键信号转导通路,最终影响细胞的增殖、分化进程及细胞因子分泌谱。此外,环境内分泌干扰物可通过改变免疫细胞的表型特征与功能状态,诱发免疫系统稳态失衡,从而提升个体对过敏性疾病、哮喘及自身免疫性疾病的易感性[13]。环境内分泌干扰物暴露,尤其是在发育早期阶段,可对个体免疫应答模式产生深远影响,可能导致长期的免疫功能障碍[14]。 环境内分泌干扰物可通过扰乱免疫系统功能,诱导自身免疫反应,介导胰岛β细胞破坏,最终引发1型糖尿病(图3)。研究表明,特定环境内分泌干扰物能够显著影响T细胞、B细胞及巨噬细胞在内的关键免疫细胞功能。以双酚A为例,其暴露可导致T细胞亚群分布失衡,促进特定促炎性细胞因子的分泌。这种免疫稳态失调可削弱免疫耐受机制,进而触发自身免疫反应[15-16]。此外,部分环境内分泌干扰物也被证实可损害抗原呈递细胞的功能,导致自身抗原的异常呈递,这一过程可激活自身反应性T细胞,最终介导对胰岛β细胞的免疫攻击[17-18]。"
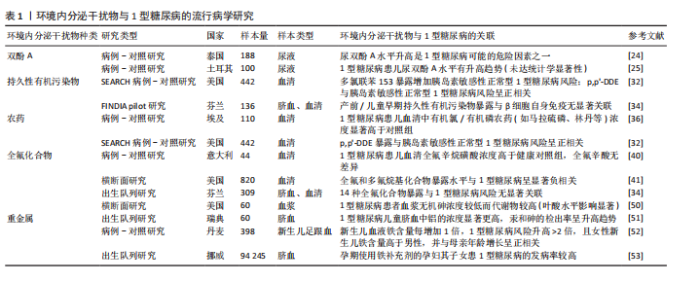
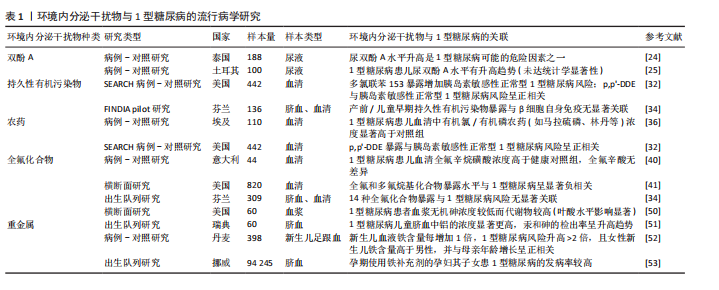
2.2.2 环境内分泌干扰物在自身免疫反应中的作用 环境内分泌干扰物在自身免疫反应中的潜在作用日益受到关注。研究表明,环境内分泌干扰物可能通过损害免疫耐受机制和干扰调节性T细胞的生成,驱动自身免疫疾病的发生与发展[14,19-20]。以双酚A为例,其暴露可通过抑制调节性T细胞增殖并促进促炎性Th17细胞分化,破坏免疫耐受平衡,进而加剧自身免疫反应,此机制为环境内分泌干扰物在1型糖尿病发病过程中的直接致病作用提供了重要的实验佐证[19-20]。值得注意的是,特定环境内分泌干扰物可扰动母体免疫系统稳态,削弱其对妊娠的免疫耐受性,从而诱发妊娠相关自身免疫反应[14]。环境内分泌干扰物在系统性红斑狼疮和类风湿关节炎等多种自身免疫疾病中也扮演着关键角色[21-22]。此外,环境内分泌干扰物还可能通过重塑细胞因子的表达谱及调控免疫细胞的活化状态,促进持续性炎症反应,最终加剧自身免疫性疾病的病理过程[23]。综上,环境内分泌干扰物不仅直接干预特定免疫细胞功能,更可能通过系统性重塑免疫稳态,协同促进自身免疫反应的启动与进展,这为深入理解环境内分泌干扰物与自身免疫疾病间的复杂关系提供了创新的理论框架。 2.3 环境内分泌干扰物与1型糖尿病的流行病学与动物实验研究进展 双酚A、持久性有机污染物、农药、全氟化合物、二噁英、邻苯二甲酸酯以及重金属等在内的多种环境内分泌干扰物与1型糖尿病发病有潜在关联,除人群流行病学证据外,相关动物实验研究亦为上述关联提供了有力佐证,下文中将对此进行整合综述。 2.3.1 双酚A与1型糖尿病 双酚A作为一种广泛用于塑料制品和食品包装的化学物质,它对1型糖尿病的潜在影响已引发广泛关注。流行病学研究提示双酚A暴露可能与1型糖尿病风险相关:一项针对泰国儿童的研究显示,1型糖尿病患者尿液中双酚A水平显著高于健康对照组,提示高双酚A暴露可能构成风险因素[24];而土耳其的一项研究虽观察到1型糖尿病患儿尿双酚A水平有升高的趋势,但此差异未达到统计学显著性[25]。动物模型研究进一步揭示了双酚A影响1型糖尿病发生的复杂机制,涉及免疫调节与肠道菌群扰动。在非肥胖糖尿病小鼠模型中,双酚A暴露表现出显著的性别差异:加速雌性小鼠1型糖尿病进程,同时延缓雄性小鼠发病,凸显性别因素在双酚A介导的1型糖尿病发病机制中的关键作用[26]。值得注意的是,双酚A的效应呈现剂量与年龄依赖性,幼年期暴露尤为关键,其诱导的促炎状态增强与1型糖尿病风险升高密切相关[27]。双酚A可诱发非肥胖糖尿病小鼠胰岛β细胞凋亡增加及组织驻留巨噬细胞数量减少,直接损害胰岛功能[28]。更深入的研究表明,自受孕起持续暴露于环境相关水平双酚A的非肥胖糖尿病小鼠,其糖尿病及胰岛炎发病率显著上升,作用机制涉及双酚A调节脾细胞和胰腺淋巴结细胞中细胞因子(如白细胞介素4、白细胞介素6、白细胞介素10、肿瘤坏死因子α和干扰素γ)的释放,重塑全身免疫系统[29];在链脲佐菌素诱导的1型糖尿病小鼠模型中,双酚A暴露(无论高低剂量)均能增加糖尿病发病率,其作用机制呈现剂量特异性:低剂量双酚A主要导致脾脏T细胞亚群减少及促炎细胞因子分泌倾向增强;而高剂量双酚A则显著调控干扰素γ和肿瘤坏死因子α水平[30]。此外,在链脲佐菌素诱导的1型糖尿病小鼠模型中,双酚A还可能通过扰乱细胞内Ca2?稳态,诱导内质网应激,进而诱发胰岛素抵抗[31]。综上,双酚A通过固有的内分泌干扰特性,以剂量依赖性方式参与1型糖尿病的发病,其作用机制多样,包括但不限于:直接干扰胰岛素分泌、损害胰腺β细胞存活与功能、重塑免疫反应(如调节T细胞活性,打破免疫耐受),最终增加自身免疫性攻击的风险。 2.3.2 持久性有机污染物与1型糖尿病 持久性有机污染物在1型糖尿病发病机制中的潜在贡献尚未完全阐明。一项纳入422名青少年糖尿病患者的病例-对照研究将参与者细分为健康对照组、胰岛素敏感性正常的1型糖尿病患者以及胰岛素抵抗的1型糖尿病患者,通过分析血浆样本中多氯联苯浓度,发现多氯联苯153暴露可显著增加胰岛素敏感性正常的1型糖尿病发病风险,而此关联在胰岛素抵抗的1型糖尿病患者中未观察到[32]。该研究进一步通过体外实验,利用环境相关浓度范围的多氯联苯153染毒INS-1E胰岛β细胞系,揭示多氯联苯153可损害β细胞在葡萄糖刺激下的胰岛素生物合成与分泌能力。研究表明,此效应与多氯联苯153显著下调关键葡萄糖感知基因(如Slc2a2和Gck)的mRNA表达水平相关[32]。此外,另有研究发现1型糖尿病儿童可能对多氯联苯138和多氯联苯153等环境内分泌干扰物诱导的甲状腺功能失调表现出易感性[33]。然而,一项出生队列研究则提示,胎儿期及生命早期暴露于持久性有机污染物并非日后发生1型糖尿病的重要风险因素[34]。动物模型为持久性有机污染物与1型糖尿病的潜在联系提供了进一步支持:研究表明持久性有机污染物混合暴露可诱发与1型糖尿病发病机制相符的代谢紊乱[35]。因此,多数研究支持持久性有机污染物在1型糖尿病病因学中扮演潜在角色,并凸显胰腺β细胞对持久性有机污染物暴露具有高度敏感性。 2.3.3 农药与1型糖尿病 农药暴露与1型糖尿病发病率之间的潜在关联日益受到研究重视。一项基于埃及人群的研究显示,75例新发1型糖尿病患儿血清中8种目标有机氯及有机磷农药的浓度显著高于健康对照组,尽管双对氯苯基三氯乙烷(DDT)、丙溴磷和甲基毒死蜱在病例组的检出率相对较低[36],这一发现提示特定农药暴露可能通过免疫系统扰动,在生命早期阶段促进1型糖尿病发生。青少年血浆中有机氯农药p,p’-DDE浓度与胰岛素敏感性正常的1型糖尿病发病风险呈显著正相关,而与伴胰岛素抵抗的1型糖尿病发病风险则无统计学显著关联[32]。体外研究证实,环境相关剂量的p,p’-DDE暴露可损害胰岛β细胞的葡萄糖刺激下的胰岛素生物合成与分泌功能,此效应与其下调关键胰岛素分泌相关基因(如Abcc8和Kcnj11)的mRNA表达水平有关[32]。然而,一项芬兰/爱沙尼亚联合研究未能确立DDE暴露与1型糖尿病或胰岛自身免疫之间的显著关联[34],凸显了研究结果的地域或人群异质性。动物模型为农药的致病作用提供了补充证据:利用黑腹果蝇模型发现,发育期暴露于有机磷杀虫剂敌敌畏可诱发胰岛素缺乏或1型糖尿病样表型,其机制可能涉及Caspase依赖性凋亡通路的激活[37]。高剂量的DDE暴露可增加非肥胖糖尿病小鼠糖尿病发病率及严重程度[38]。综上,现有证据多支持特定农药暴露作为1型糖尿病发病的重要潜在风险因素,其作用机制涉及对胰岛β细胞功能的直接损害以及对免疫耐受的破坏。 2.3.4 全氟化合物与1型糖尿病 全氟化合物作为一类广泛应用于防水、防油和防污产品的化学物质,其代表性成员包括全氟辛烷磺酸(PFOS)、全氟辛酸(PFOA)、全氟己烷磺酸(PFHxS)、全氟壬酸(PFNA)、全氟癸酸(PFDA)以及全氟十一烷酸(PFUnDA)等。关于全氟化合物暴露与1型糖尿病的关联性,现有流行病学研究呈现显著分歧。一项母婴队列研究揭示,较高的全氟化合物暴露水平与脐血中磷脂水平降低以及1型糖尿病相关的胰岛自身抗体的检出存在关联[39]。意大利的一项研究观察到,新发1型糖尿病患儿血清全氟辛烷磺酸水平高于健康对照组,而全氟辛酸水平则未见显著差异[40]。与此形成鲜明对比的是,一项纳入820名1型糖尿病患者的美国横断面研究发现,较高水平的全氟辛酸、全氟辛烷磺酸、全氟己基磺酸和全氟壬酸与较低的1型糖尿病风险显著相关[41]。而一项出生队列研究则未发现14种全氟化合物暴露构成1型糖尿病的显著风险因素[34]。综上,当前关于全氟化合物与1型糖尿病关联性的研究结论尚不一致。值得注意的是,现有研究多聚焦于表型层面的关联分析,关于全氟化合物如何通过诱导代谢-免疫交互网络失衡进而参与1型糖尿病发病的具体分子机制,仍有待深入探索。 2.3.5 二噁英与1型糖尿病 二噁英作为一类普遍存在且高度稳定的环境污染物,与多种健康问题的关联已获研究证实。然而,关于二噁英与1型糖尿病发病风险的人群流行病学相关性,目前尚缺乏系统性研究。在非肥胖糖尿病小鼠模型中,长期给予2,3,7,8-四氯二苯并二噁英(TCDD)进行干预,可显著抑制自身免疫性1型糖尿病的进展,该效应伴随胰腺胰岛炎程度明显减轻,以及胰腺引流淋巴结中CD4+CD25+Foxp3+Tregs群体显著扩增。值得注意的是,若在第15周终止2,3,7,8-四氯二苯并二噁英处理,小鼠则会在后续8周内迅速进展为1型糖尿病,强烈提示该免疫抑制效应依赖于芳香烃受体的持续性激活[42]。机制研究揭示,二噁英通过激活芳香烃受体,抑制多种T细胞和B细胞的免疫反应;芳香烃受体配体可触发下游表观遗传调控网络,从而参与炎症反应调节和自身免疫性疾病的潜在治疗[43]。然而,2,3,7,8-四氯二苯并二噁英对免疫系统的影响呈现高度复杂性。值得注意的是,孕期暴露于二噁英可能促进小鼠成年期自身免疫性疾病的发生发展[44],亦提示其对1型糖尿病的潜在致病作用。 2.3.6 邻苯二甲酸酯与1型糖尿病发病率的相关性研究 作为塑料增塑剂的代表性环境内分泌干扰物,邻苯二甲酸酯与1型糖尿病的致病关联已成为代谢性疾病研究的前沿焦点。葡萄牙一项探讨邻苯二甲酸酯与1型糖尿病关系的流行病学研究揭示,相较于健康对照,新发及已确诊1型糖尿病患儿尿液中邻苯二甲酸二异丁酯(DiBP)的主要代谢产物——单异丁基邻苯二甲酸酯(MiBP)的浓度较高,但该差异未达统计学显著性[45]。此外,比利时学者针对54例1型糖尿病儿童的研究发现,特定邻苯二甲酸酯代谢物与糖化血红蛋白水平呈正相关[33]。现有证据提示,邻苯二甲酸酯可能通过“代谢-免疫双重打击”机制参与1型糖尿病病理进程:在代谢调控层面,孕期暴露于邻苯二甲酸二乙基己酯可显著改变子代大鼠葡萄糖代谢模式[46],其直接暴露则通过诱导线粒体功能障碍,驱动胰腺β细胞凋亡并损害胰岛素分泌动力学[47];在免疫调控层面,邻苯二甲酸二丁酯的毒理学研究证实,该物质不仅能异常激活免疫系统 [48],在甲状腺炎等典型自身免疫疾病模型中更展现出明确的致病作用[49]。 2.3.7 重金属与1型糖尿病 近年来研究提示,孕期重金属暴露可能是影响儿童1型糖尿病发病风险的重要环境因素。美国一项横断面研究数据显示,相较于健康个体,1型糖尿病患者血浆中无机砷水平较低,而其代谢产物水平较高,该现象在叶酸水平较高的人群中尤为突出[50],这表明砷的体内代谢过程可能参与1型糖尿病的病理进程,且环境砷暴露与叶酸等营养因素之间存在潜在交互作用,共同调控疾病易感性[50]。瑞典ABIS出生队列数据显示,未来发展为1型糖尿病的儿童,其脐血中铝浓度显著高于健康对照,汞和砷的检出率亦呈现非显著性升高趋势 [51]。丹麦一项病例-对照研究发现,新生儿血液铁含量每增加1倍,其未来1型糖尿病发病风险升高逾2倍;值得注意的是,女性新生儿铁含量高于男性,且与母亲年龄增长呈正相关[52]。研究推测,铁过载可能通过氧化应激破坏胰岛β细胞功能或调节免疫反应参与1型糖尿病发病。挪威母亲和儿童队列研究进一步证实,孕期铁补充与后代1型糖尿病风险升高相关。机制探索发现,孕期铁补充剂的使用与脐血中铁状态生物标志物水平升高有关,而后者与1型糖尿病风险增加存在关联[53]。遗传学证据亦支持该关联:携带增加铁储存风险的HFE基因变异的母亲,其后代罹患1型糖尿病的风险显著增高[53]。综合上述研究,孕期铁过载可能通过诱导胎儿氧化应激(损害胰岛β细胞)和/或干扰免疫系统发育,进而增加1型糖尿病的发病风险。动物模型研究为机制研究提供线索,无机汞暴露可诱发胰岛β细胞氧化应激和细胞凋亡,抑制胰岛素分泌,并扰乱免疫稳态,值得注意的是,抗氧化剂N-乙酰半胱氨酸可逆转无机汞诱导的胰岛素分泌功能障碍[54]。在非肥胖糖尿病小鼠模型中,低剂量砷暴露显著上调胰岛β细胞中miR-2909的表达,miR-2909通过靶向抑制PDX1和PI3K基因表达,导致胰岛素转录合成障碍[55],现有证据提示,重金属暴露,特别是在生命早期可能通过干扰β细胞功能/胰岛素合成、诱发氧化应激、调节免疫反应及表观遗传改变等多重机制参与1型糖尿病的发病。然而,精确的分子通路和人群归因风险仍需深入探究。 表1系统总结了近年来发表的环境内分泌干扰物与1型糖尿病关联性的重要流行病学研究进展。"
| [1] HUANG S, LI F, QUAN C, et al. Intestinal flora: a potential pathogenesis mechanism and treatment strategy for type 1 diabetes mellitus. Gut Microbes. 2024;16(1):2423024. [2] IKEGAMI H, BABAYA N, NOSO S, et al. β-Cell failure in diabetes: Common susceptibility and mechanisms shared between type 1 and type 2 diabetes. J Diabetes Investig. 2021;12(9):1526-1539. [3] AHN C, JEUNG EB. Endocrine-Disrupting Chemicals and Disease Endpoints. Int J Mol Sci. 2023;24(6):5342. [4] LIN S, LI J, YAN X, et al. Maternal pesticide exposure and risk of preterm birth: A systematic review and meta-analysis. Environ Int. 2023;178:108043. [5] CHEN Y, YANG J, YAO B, et al. Endocrine disrupting chemicals in the environment: Environmental sources, biological effects, remediation techniques, and perspective. Environ Pollut. 2022;310:119918. [6] TUMU K, VORST K, CURTWILER G, et al. Endocrine modulating chemicals in food packaging: A review of phthalates and bisphenols. Compr Rev Food Sci Food Saf. 2023;22(2):1337-1359. [7] MAHDAVIANPOUR M, CHAMKOURI N, CHAMKOURI H, et al. Determination of bisphenol a migration from food packaging by dispersive liquid-liquid microextraction. MethodsX. 2021;8:101415. [8] PREDIERI B, IUGHEZZI L, BERNASCONI S, et al. Endocrine Disrupting Chemicals’ Effects in Children: What We Know and What We Need to Learn? Int J Mol Sci. 2022;23(19):11899. [9] DUTTA S, RUDEN DM. Heavy Metals in Umbilical Cord Blood: Effects on Epigenetics and Child Development. Cells. 2024;13(21):1775. [10] JAIN J, GUPTA N, MATHUR R, et al. A Study on Impact of BPA in the Adipose Tissue Dysfunction (Adiposopathy) in Asian Indian Type 2 Diabetes Mellitus Subjects. Indian J Clin Biochem. 2020;35(4):451-457. [11] ALY HM, IBRAHEEM RB, MAHMOUD RM, et al. The Relationship Between Polychlorinated and Polybrominated Biphenyls and Glycated Hemoglobin among Electronics Workers. Indian J Occup Environ Med. 2024;28(2):143-147. [12] NOWAK K, JABŁONSKA E, RATAJCZAK-WRONA W, et al. Immunomodulatory effects of synthetic endocrine disrupting chemicals on the development and functions of human immune cells. Environ Int. 2019;125:350-364. [13] CASAS M, GASCON M. Prenatal Exposure to Endocrine-Disrupting Chemicals and Asthma and Allergic Diseases. J Investig Allergol Clin Immunol. 2020;30(4):215-228. [14] SCHJENKEN JE, GREEN ES, OVERDUIN TS, et al. Endocrine Disruptor Compounds-A Cause of Impaired Immune Tolerance Driving Inflammatory Disorders of Pregnancy? Front Endocrinol (Lausanne). 2021;12:607539. [15] HOWARD SG. Developmental Exposure to Endocrine Disrupting Chemicals and Type 1 Diabetes Mellitus. Front Endocrinol (Lausanne). 2018;9:513. [16] ALJADEFF G, LONGHI E, SHOENFELD Y. Bisphenol A: A notorious player in the mosaic of autoimmunity. Autoimmunity. 2018;51(8):370-377. [17] VIDAL OS, DEEPIKA D, SCHUHMACHER M, et al. EDC-induced mechanisms of immunotoxicity: a systematic review. Crit Rev Toxicol. 2021;51(7):634-652. [18] ZHANG Q, LI M, WANG P, et al. Integrated analysis reveals the immunotoxicity mechanism of BPs on human lymphocytes. Chem Biol Interact. 2024;423:107872. [19] GAO L, LUO D, WU D, et al. Effects of mammalian target of rapamycin and aryl hydrocarbon receptor-mediating autophagy signaling on the balance of Th17/Treg cells during perinatal bisphenol A exposure in female offspring mice. Environ Toxicol. 2022;37(7):1781-1789. [20] WANG S, DONG Y, ZHAI L, et al. Decreased Treg cells induced by bisphenol A is associated with up-regulation of PI3K/Akt/mTOR signaling pathway and Foxp3 DNA methylation in spleen of adolescent mice. Chemosphere. 2024;357:141957. [21] HUANG RG, Li XB, WANG YY, et al. Endocrine-disrupting chemicals and autoimmune diseases. Environ Res. 2023; 231(Pt 2):116222. [22] WANG Y, WU H, LI K, et al. Environmental triggers of autoimmunity: The association between bisphenol analogues and systemic lupus erythematosus. Ecotoxicol Environ Saf. 2024;278:116452. [23] FISCHER F, ERMER MR, HOWANSKi J, et al. Single and mixture effects of bisphenol A and benzophenone-3 on in vitro T helper cell differentiation. Chem Biol Interact. 2024;395:111011. [24] TOSIRISUK N, SAKORN N, JANTARAT C, et al. Increased bisphenol A levels in Thai children and adolescents with type 1 diabetes mellitus. Pediatr Int. 2022;64(1):e14944. [25] INCE T, BALCI A, YALÇIN S, et al. Urinary bisphenol-A levels in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2018;31(8):829-836. [26] XU J, HUANG G, NAGY T, et al. Sex-dependent effects of bisphenol A on type 1 diabetes development in non-obese diabetic (NOD) mice. Arch Toxicol. 2019;93(4):997-1008. [27] XU J, HUANG G, NAGY T, et al. Bisphenol A alteration of type 1 diabetes in non-obese diabetic (NOD) female mice is dependent on window of exposure. Arch Toxicol. 2019; 93(4):1083-1093. [28] HONG Y, WANG D, LIN Y, et al. Environmental triggers and future risk of developing autoimmune diseases: Molecular mechanism and network toxicology analysis of bisphenol A. Ecotoxicol Environ Saf. 2024;288:117352. [29] BODIN J, KOCBACH BÖLLING A, WENDT A, et al. Exposure to bisphenol A, but not phthalates, increases spontaneous diabetes type 1 development in NOD mice. Toxicol Rep. 2015;2:99-110. [30] CETKOVIC-CVRLJE M, THINAMANY S, BRUNER KA. Bisphenol A (BPA) aggravates multiple low-dose streptozotocin-induced type 1 diabetes in C57BL/6 mice. J Immunotoxicol. 2017;14:160-168. [31] AHN C, KANG HS, LEE JH, et al. Bisphenol A and octylphenol exacerbate type 1 diabetes mellitus by disrupting calcium homeostasis in mouse pancreas. Toxicol Lett. 2018;295:162-172. [32] BRESSON SE, ISOM S, JENSEN ET, et al. Associations between persistent organic pollutants and type 1 diabetes in youth. Environ Int. 2022;163:107175. [33] DUFOUR P, PIRARD C, LEBRETHON MC, et al. Associations between endocrine disruptor contamination and thyroid hormone homeostasis in Belgian type 1 diabetic children. Int Arch Occup Environ Health. 2023;96(6):869-881. [34] SALO HM, KOPONEN J, KIVIRANTA H, et al. No evidence of the role of early chemical exposure in the development of -cell autoimmunity. Environ Sci Pollut Res Int. 2019;26:1370-1378. [35] SINIOJA T, BODIN J, DUBERG D, et al. Exposure to persistent organic pollutants alters the serum metabolome in non-obese diabetic mice. Metabolomics. 2022; 18(11):87. [36] EL-MORSI DA, RAHMAN RH, ABO-ARAB AA. Pesticides residues in Egyptian diabetic children: A preliminary study. J Clin Toxicol. 2012;2:138-142. [37] GUPTA HP, JHA RR, AHMAD H, et al. Xenobiotic mediated diabetogenesis: Developmental exposure to dichlorvos or atrazine leads to type 1 or type 2 diabetes in Drosophila. Free Radic Biol Med. 2019; 141:461-474. [38] CETKOVI-CYRLJE M, OLSON M, SCHINDLER B, et al. Exposure to DDT metabolite p,p’-DDE increases autoimmune type 1 diabetes incidence in NOD mouse model. J Immunotoxicol 2016;13:1-11. [39] MCGLINCHFY A, SINIOJA T, LAMICHHANE S, et al. Prenatal exposure to perfluoroalkyl substances modulates neonatal serum phospholipids, increasing risk of type 1 diabetes. Environ Int. 2020;143:105935. [40] PREDIERI B, IUGHEZZI L, GUERRANTI C, et al. High levels of perfluorooctane sulfonate in children at the onset of diabetes. Int J Endocrinol. 2015;2015:234358. [41] CONWAY B, INNES KE, LONG D. Perfluoroalkyl substances and beta cell deficient diabetes. J Diabetes Complications. 2016;30(6):993-998. [42] KERKVLIET NI, STEPPAN LB, VORACHEK W, et al. Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes. Immunotherapy. 2009;1(4):539-547. [43] PRASAD SINGH N, NAGARKATTI M, NAGARKATTI P, et al. From Suppressor T cells to Regulatory T cells: How the Journey That Began with the Discovery of the Toxic Effects of TCDD Led to Better Understanding of the Role of AhR in Immunoregulation. Int J Mol Sci. 2020;21(21):7849. [44] MUSTAFA A, HOLLADAY SD, WITONSKY S, et al. A single mid-gestation exposure to TCDD yields a postnatal autoimmune signature, differing by sex, in early geriatric C57BL/6 mice. Toxicology. 2011;290(2-3):156-168. [45] CASTRO-CORREIA C, CORREIA-SÁ L, NORBERTO S, et al. Phthalates and type 1 diabetes: is there any link? Environ Sci Pollut Res Int. 2018;25(18):17915-17919. [46] LIN Y, WEI J, Li Y, et al. Developmental exposure to di(2-ethylhexyl) phthalate impairs endocrine pancreas and leads to long-term adverse effects on glucose homeostasis in the rat. Am J Physiol Endocrinol Metab. 2011;301:E527-E538. [47] SUN Y, LIN Q, HUANG Q, et al. Di(2-ethylhexyl) phthalate-induced apoptosis in rat INS-1 cells is dependent on activation of endoplasmic reticulum stress and suppression of antioxidant protection. J Cell Mol Med. 2015;19(3):581-594. [48] LOURENÇO AC, GALBIATI V, CORTI D, et al. The plasticizer dibutyl phthalate (DBP) potentiates chemical allergen-induced THP-1 activation. Toxicol in Vitro. 2015;29(8):2001-2008. [49] WU Y, LI J, YAN B, et al. Oral exposure to dibutyl phthalate exacerbates chronic lymphocytic thyroiditis through oxidative stress in female Wistar rats. Sci Rep. 2017;7: 15469. [50] LUDVIGSSON J, ANDERSSON-WHITE P, GUERRERO-BOSAGNA C, et al. Toxic metals in cord blood and later development of Type 1 diabetes. Pediatr Dimens. 2019;4(2): 10.15761/PD.1000186. [51] KYVSGAARD JN, OVERGAARD AJ, THORSEN SU, et al. High Neonatal Blood Iron Content Is Associated with the Risk of Childhood Type 1 Diabetes Mellitus. Nutrients. 2017; 9(11):1221. [52] GRAU-PÉREZ M, KUO CC, SPRATLEN M, et al. The Association of Arsenic Exposure and Metabolism With Type 1 and Type 2 Diabetes in Youth: The SEARCH Case-Control Study. Diabetes Care. 2017;40(1):46-53. [53] STØRDAL K, MCARDLE HJ, HAYES H, et al. Prenatal iron exposure and childhood type 1 diabetes. Sci Rep. 2018;8(1):9067. [54] CHEN YW, HUANG CF, YANG CY, et al. Inorganic mercury causes pancreatic beta-cell death via the oxidative stress-induced apoptotic and necrotic pathways. Toxicol Appl Pharmacol. 2010;243(3): 323-331. [55] RAMDAS M, SHARMA S, KAUL D, et al. Possible role of miR-2909 RNomics in arsenic mediated pancreatic β-cell dysfunction. J Trace Elem Med Biol. 2018; 50:263-267. |
| [1] | Shi Yaozhou, Jia Fanglin, Zhang Heling, Song Hanlin, Gao Haoran, Gao Xiao, Sun Wei, Feng Hu. Establishment and validation of a prediction model for axial symptoms after laminectomy with lateral mass screw fixation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2269-2277. |
| [2] | Zhang Zizheng, Luo Wang, Liu Changlu. Application value of finite element analysis on unicompartmental knee arthroplasty for medial knee compartmental osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2313-2322. |
| [3] | Zhao Feifan, Cao Yujing. Risk factors and coping strategies of internal fixation failure in treatment of intertrochanteric fracture with proximal femoral nail antirotation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2323-2333. |
| [4] | Li Qingbin, Lin Jianhui, Huang Wenjie, Wang Mingshuang, Du Jiankai, Lao Yongqiang. Bone cement filling after enlarged curettage of giant cell tumor around the knee joint: a comparison of subchondral bone grafting and non-grafting [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1896-1902. |
| [5] | Abuduwupuer·Haibier, Shang Qisong Song Xinghua. Analysis of factors for recurrent fractures of vertebral and adjacent vertebrae after osteoporotic compression fracture in the elderly patients with underlying diseases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 642-651. |
| [6] | Yang Peng, Xu Chenghan, Zhou Yingjie, Chai Xubin, Zhuo Hanjie, Li Lin, Shi Jinyu. A meta-analysis of risk factors for residual back pain after vertebral augmentation for osteoporotic vertebral compression fractures [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 731-739. |
| [7] | Hu Yalin, Huang Fengqin, Yang Boyin, Luo Xingmei. Transcription factor EB improves Alzheimer’s disease via the autophagy-lysosome pathway [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5844-5858. |
| [8] | Zhang Junwei, Chen Lingling, Ma Zhenyuan, Nie Weizhi, Li Chaohui, Wang Haitao, Duan Laibao, Hou Jinyong, Bi Hongzheng. Three-dimensional displacement and risk factors of midshaft clavicle fractures treated with titanium elastic intramedullary nailing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 269-277. |
| [9] | Xu Feng, Gu Dongyang, Zhu Zihao, Li Qiujie, Wan Xianglin. Relationship between spatio-temporal gait characteristics and fall risk in stroke patients [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4038-4044. |
| [10] | La Rui, Wu Qian, Zhang Zhongtai, Xu Wu, Ding Qingfeng, Zhang Zhigang, Jiang Dinghua, Huang Lixin, Wang Shenghao. Analysis of risk factors for secondary fractures after hip fracture surgery in the elderly [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(15): 3920-3928. |
| [11] | Ma Le, Song Yuke, Zhong Xianxing, Zhang Wensheng. Risk factors for axial symptoms following posterior cervical laminoplasty: a systematic review and meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(15): 3983-3992. |
| [12] | Yang Xirui, He Jinfeng. Pathogenesis of diabetic periodontitis and its local drug delivery treatment strategies [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2846-2857. |
| [13] | Yan Wenjian, Li Yinghui, Zhang Yong. Daily diet and structural damage of the knee joint: a large-scale genetic analysis based on UK and FinnGen databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2877-2885. |
| [14] | Huang Fengqin, Hu Yalin, Yang Boyin, Luo Xingmei. Constructing a risk prediction nomogram model for cognitive impairment in hypertensive intracerebral hemorrhage [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2466-2474. |
| [15] | Jiang Kai, Rong Yifa, Jia Haifeng, Li Hanzheng, Lu Bowen, Liang Xuezhen, Li Gang. Relationship between inflammatory factors and rheumatoid arthritis: a large-sample analysis based on the FinnGen R10 database and genome-wide association studies [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2629-2640. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||