Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (23): 5972-5981.doi: 10.12307/2026.347
Previous Articles Next Articles
Glycocalyx: the new link between exercise and disease
Ma Zhennan, Wang Yinfeng, Yao Lijuan, Chen Leqin
- Shanxi Normal University, Taiyuan 030031, Shanxi Province, China
-
Received:2025-05-19Accepted:2025-08-05Online:2026-08-18Published:2025-12-31 -
Contact:Chen Leqin, PhD, Professor, Shanxi Normal University, Taiyuan 030031, Shanxi Province, China -
About author:Ma Zhennan, MS, Shanxi Normal University, Taiyuan 030031, Shanxi Province, China
CLC Number:
Cite this article
Ma Zhennan, Wang Yinfeng, Yao Lijuan, Chen Leqin. Glycocalyx: the new link between exercise and disease[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 5972-5981.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
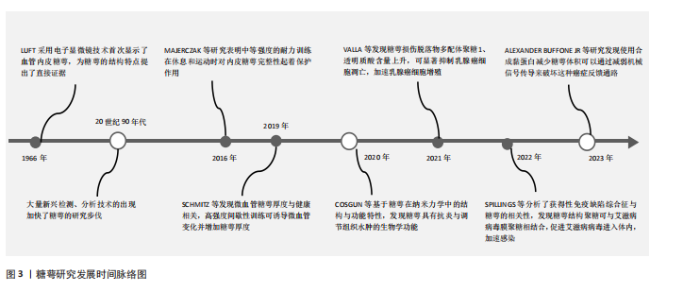
2.1 糖萼的基本定义 2.1.1 糖萼的结构 糖萼属于一种动态生化结构体系,主要由蛋白聚糖、糖胺聚糖侧链、糖脂复合物及唾液酸蛋白等生物大分子构成[3]。糖萼为带负电荷的凝胶状层状体系,具有显著的抗粘连及抗凝特性,广泛分布于血管内皮细胞表面[4]。糖胺聚糖和蛋白聚糖是糖萼的重要组成成分[5]。 糖胺聚糖作为一类具有显著负电特征的酸性多糖大分子,基本结构为含可变硫酸化位点的二糖单元,通过α/β构型糖苷键共价聚合形成无支链酸性多糖,构成糖萼结构的核心主体[6-8]。糖胺聚糖分布于动物组织的细胞膜表面和细胞外基质中,主要功能为参与胚胎发育调控、跨膜信号转导级联、促进神经元轴突定向延伸、促血管新生、介导肿瘤细胞侵袭转移、抑制炎症反应及调控凝血级联反应等特性[9-12]。 蛋白聚糖作为一类特殊的糖蛋白,由一个核心蛋白与一种或多种糖胺聚糖相连而成,依靠核心蛋白(如多配体聚糖、蛋白多糖等)上的特定基团与细胞膜相结合,从而成为糖萼结构的关键“骨架”分子[13-15]。由于蛋白聚糖的组成成分中包含糖胺聚糖,因此蛋白聚糖与糖胺聚糖在功能上存在部分重合。蛋白聚糖在多个生理过程中发挥关键调控作用:参与神经元轴突导向及极性形成过程;针对紧密连接蛋白的调控策略可有效维护血脑屏障的结构稳态;介导细胞表面整合素与基质蛋白的相互作用,从而对细胞迁移和黏附进行精密调控。在机体防御机制方面,蛋白聚糖可抵御外源性有害因子侵袭,发挥脏器保护作用,对肾脏的保护效应尤为明显,具体表现为改善肾小球基底膜选择通透性、降低尿蛋白排泄量[16-17]。 多配体聚糖通过其核心蛋白骨架的特殊结构,在维持血管内皮糖萼结构稳定性方面发挥关键作用[18]。值得注意的是,与血浆蛋白的结合模式不同,糖胺聚糖通过其表面特异性配体与多种生物活性分子相互作用,哪怕是浓度与结构发生细小的变动也会引发糖萼屏障功能的改变[19]。 2.1.2 糖萼研究发展时间脉络 糖萼研究发展时间脉络[20-24],见图3。 2.2 糖萼的功能 糖萼作为血管内皮的生物屏障,在调控血管的通透平衡、介导炎症反应、血液剪切应力传感器、抗凝等起着关键作用。 2.2.1 屏障功能 糖萼紧密覆盖在内皮细胞的胞间连接区域,在维持屏障功能方面起着至关重要作用。高度硫酸化的糖胺聚糖通过其分子屏障特性能够选择性地阻止带负电荷及分子质量超过70 ku的物质进入内皮细胞,这种特性对于限制白蛋白的跨细胞流动起到了关键作用,进而维持血管腔两侧的胶体渗透压梯度,有利于管腔对液体的重吸收[25],揭示了血管内皮糖萼在微循环稳态中扮演关键调控角色。相关研究表明,肾小球滤过屏障效能下降与糖萼结构损伤存在显著正相关[26-27]。有研究通过对内皮细胞表面层中不同血浆标记分子的通透性检测发现,糖萼层电荷选择特性的改变可显著影响血管壁的通透性[28]。 2.2.2 血液机械力传感器 血管内皮细胞常常暴露于血流的机械力,剪切应力的暴露导致内皮细胞产生一氧化氮(NO),这是血管张力的重要决定因素,糖萼在这一过程中扮演着内皮细胞剪切应力机械传感器的角色。研究表明,硫酸乙酰肝素与透明质酸在机械-化学信号转导过程中发挥分子换能器作用[29]。内皮细胞能够通过瞬时受体电位通道摄取Ca2+,促进NO的合成释放。HU等[30]在肝素酶环境中对人脐静脉血管内皮细胞进行实验,结果发现当人脐静脉血管内皮细胞中的硫酸乙酰肝素缺失时,细胞在流体剪切应力作用下的迁移速率提升,这说明糖萼具备充当剪切应力传感器的功能,能够对剪切力的变化作出响应。 2.2.3 调节炎症反应 在炎症反应进程中,糖胺聚糖通过其硫酸化修饰位点与趋化因子相互作用,这种相互作用促使炎症部位构建起趋化因子的触觉梯度或固定梯度,诱导产生“趋化因子云”现象,这一变化导致糖萼界面内趋化因子的浓度呈现非均匀分布。该梯度场的形成进一步激活了趋化因子与白细胞表面整合素的结合,推动白细胞活化,最终实现白细胞跨膜迁移效率的调控[31]。糖胺聚糖中的硫酸乙酰肝素能够作为L-选择素的特异性配体,参与白细胞在血管内的跨膜迁移力学-化学耦合。不仅如此,硫酸乙酰肝素还具备调节趋化因子浓度梯度的能力,通过这一调节机制促使内皮细胞与白细胞紧密黏附。 此外相关研究结果显示,因炎症导致的糖萼脱落会加快单核细胞的黏附速度,促使巨噬细胞浸润到存在脂质潴留的区域,这些巨噬细胞也会受此影响进一步参与炎症反应进程[32]。"
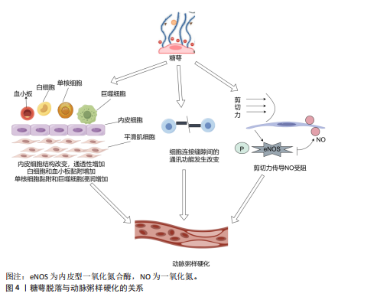
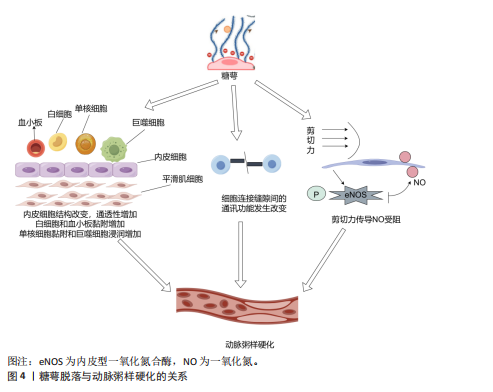
2.2.4 抗凝作用 血管内皮糖萼是维持血管屏障的重要结构,在正常生理条件下该结构通过形成阻隔层防止血细胞与内皮直接接触,进而降低血栓形成的风险;此外,糖萼还可与抗凝血酶Ⅲ、血栓调节蛋白及组织因子途径抑制物产生分子相互作用。糖萼调控凝血过程的作用机制主要涉及以下关键环节:抗凝血酶Ⅲ通过特异性结合糖萼中的硫酸乙酰肝素,显著提升其抑制凝血因子的生物活性;血栓调节蛋白与硫酸软骨素结合后促进凝血酶向蛋白C激活剂转化,形成负反馈调节机制;组织因子途径抑制剂TFPI作为凝血级联反应中FⅦa/FXa复合物的特异性抑制剂,通过糖萼表面硫酸乙酰肝素的分子互作介导凝血调控作用[33]。 2.3 糖萼与疾病的关联 2.3.1 糖萼与心血管疾病的关联性 糖萼的完整状态对机体维持正常血液循环的稳定以及保障各脏器的生理功能起着不可或缺的关键作用。血管内皮糖萼脱落引发一系列病理效应,具体表现为:内皮屏障完整性受损导致血管通透性异常增高;血管舒缩调节功能紊乱引发血流动力学异常;白细胞-内皮细胞黏附分子表达上调诱发炎症反应;凝血-抗凝平衡失调促使血栓形成,最终危及血管稳态;诱发血管炎症,进一步扰乱血管的正常生理环境[34]。 糖萼脱落可引发内皮屏障结构改变,导致内皮通透性异常增加,这一病理反应不仅促进血管壁脂质蓄积及炎症因子释放,同时通过单核细胞-内皮黏附驱动巨噬细胞跨内膜迁移,引发泡沫细胞病理性生成,上述反应共同加速动脉粥样硬化病理进程[35];此外,糖萼脱落会抑制细胞间正常通讯、阻碍剪切力传导,进一步加剧动脉粥样硬化斑块的形成,见图4。 诸多研究表明,糖萼在动脉粥样硬化的发生机制及病理进展中发挥关键作用。例如,MIRANDA等[36]发现急性冠状动脉综合征患者发病12 h后可检测到多配体聚糖1的血浆浓度升高,这表明糖萼降解与急性冠状动脉综合征疾病进程存在显著病理关联。NEMOTO等[37]证实糖萼结构完整性破坏与薄纤维帽动脉粥样硬化斑块存在显著关联。血清多配体聚糖1浓度变化可作为评估斑块易损性的指标。CANCEL等[38]的研究表明,糖萼结构完整性丧失与动脉粥样硬化存在密切关联,糖萼脱落通过激活单核细胞向内皮迁移信号通路并诱导巨噬细胞浸润,导致血管壁脂质异常蓄积,从而加速动脉粥样硬化斑块的产生。糖萼动态平衡调控在动脉粥样硬化斑块形成中具有枢纽作用。综上所述,靶向调控糖萼或为动脉粥样硬化防治提供新的精准干预靶点。 2.3.2 糖萼与癌症的关联性 癌症的发生机制极为复杂,病死率长期居高不下,攻克这一难题已然成为全球科研领域的焦点。近些年来,部分学者将糖萼与癌症相关联,从糖生物学这一独特视角切入,深度挖掘癌症发生的内在机制,为癌症研究开辟新的路径与方向。肿瘤微环境中细胞外基质与邻近组织间的机械相互作用证实,这种机械信号传导通路可显著调控细胞增殖、迁移及分化等多种功能,并参与肿瘤相关病理生理过程。肿瘤-细胞外基质相互作用的介质是糖萼,它在肿瘤和细胞外基质之间充当缓冲器[39]。 HOJMAN等[40]发现乳腺癌细胞增殖会被由运动诱导产生的骨骼肌源性肌球蛋白抑制,该发现为运动抗癌理论提供了新靶点。VALLA等[41]发现糖萼损伤脱落物(糖胺聚糖/蛋白聚糖)在调控三阴性乳腺癌细胞中发挥关键作用,多配体聚糖1与透明质酸的含量升高可抑制肿瘤细胞程序性死亡,同时伴随细胞周期调控异常,加速乳腺癌细胞恶性增殖。对糖萼在肿瘤细胞中的表达实施靶向修饰,或可为恶性肿瘤治疗提供新型干预策略。MORAN等[42]研究发现,肾癌细胞转移活性与糖萼组分硫酸乙酰肝素、透明质酸合成水平呈正相关,在重度联合免疫缺陷小鼠模型中,硫酸乙酰肝素、透明质酸表达下调可显著抑制肿瘤转移进程,基于此,他们通过靶向调控透明质酸合酶基因表达推进抗肾癌转移的治疗。TONDEPU等[43]基于胶质母细胞瘤微环境中糖萼及糖胺聚糖基质的关键病理特征,设计出一种3D糖质,该3D糖质能够对胶质母细胞瘤的病情与治疗效果进行精准评估,为开发靶向糖萼的治疗策略提供了全新平台。MIRANDA 等[36]研究发"
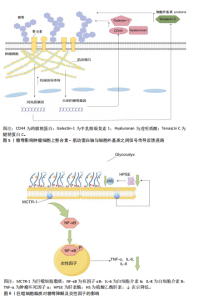
现,糖萼能够驱动肿瘤细胞上整合素-肌动蛋白轴与细胞外基质之间增强的机械信号传导,这种机械信号传导促使细胞核中的基因上调,从而在肿瘤中驱动产生更具间充质表型(产生细胞外基质蛋白,如腱糖蛋白C)和更厚的糖萼(如CD44、透明质酸和半乳糖凝集素1),这些因素在一个依赖张力的反馈回路中驱动胶质瘤的侵袭性,并且该回路具有自我增强的特性。糖萼影响肿瘤细胞上整合素-肌动蛋白轴与细胞外基质之间信号传导反馈通路,如图5所示。 2021年,OLIVEIRA 等[44]研究发现糖胺聚糖表现出明显的整体重构特性,同时与MLL-r细胞分化过程中的特定阶段存在密切关联。这一重要发现证实了糖萼成分的结构变化与血液系统恶性肿瘤发展之间具有显著生物学联系,为相关病理机制研究提供了新的科学依据。 癌细胞表面糖萼的广泛增厚及损伤脱落产物促进了肿瘤增殖、转移扩散及疾病恶化。保持定期运动的习惯能够降低癌症的复发风险及死亡率,但仍面临许多挑战。 2.3.3 糖萼与抗炎的关联性 研究显示炎症反应的初始作用靶点集中于血管内皮糖萼,糖萼结构完整性的破坏已被证实与炎症发生发展存在密切关联[45]。COVID-19是一种病毒性肺炎,病原体为SARS-CoV-2[46]。SARS-CoV-2病毒的自然宿主主要为鸟类及哺乳动物,该病毒感染机体后可通过系统性炎症反应导致多器官功能障碍,而在这些反应中呼吸系统所受的影响最为显著和剧烈[47]。2021年,LAMBADIARI等[48]研究发现COVID-19病毒感染可诱导内皮糖萼发生形态学改变,进而导致糖萼功能受损。QUEISSER 等[49]研究表明,当血管内皮糖萼完整性受损时,糖萼损伤脱落物可进入血液循环系统导致COVID-19患者的炎症反应加剧。PREEZ等[50]发现,糖萼结构完整状态下的表面负电荷屏障可通过与SARS-CoV-2病毒形成互斥,显著降低病毒与宿主细胞的黏附效率,从而抑制病毒入侵人体。 MASOLA等[51]、SONG等[52]研究揭示了炎症与糖萼降解存在关联,巨噬细胞在炎症刺激下通过Toll样受体介导核因子κB信号通路活化,这一过程促进肿瘤坏死因子α、白细胞介素6及白细胞介素8等促炎因子表达。其中,肿瘤坏死因子α通过激活肥大细胞脱颗粒反应释放蛋白酶、组胺及硫酸乙酰肝素酶等物质,这些物质的释放导致糖萼降解。值得注意的是,哺乳动物中肝素酶作为唯一可降解硫酸乙酰肝素的内切糖苷酶[53],在脓毒症时中呈现显著性升高[54]。LI等[55]研究发现,在脂多糖诱导的脓毒症中,巨噬细胞的脂质通过调节核因子κB信号通路活性显著缓解肺毛细血管内皮细胞糖萼的结构损伤,分子保护机制包括:下调肝素酶表达,阻断硫酸乙酰肝素的分解代谢;抑制核因子κB p65的磷酸化进程,降低肿瘤坏死因子α、白细胞介素6等炎性因子释放;发挥对糖萼的保护作用,见图6。"
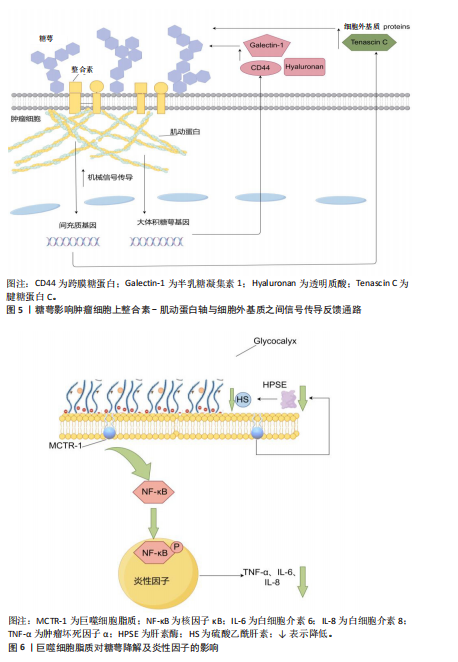
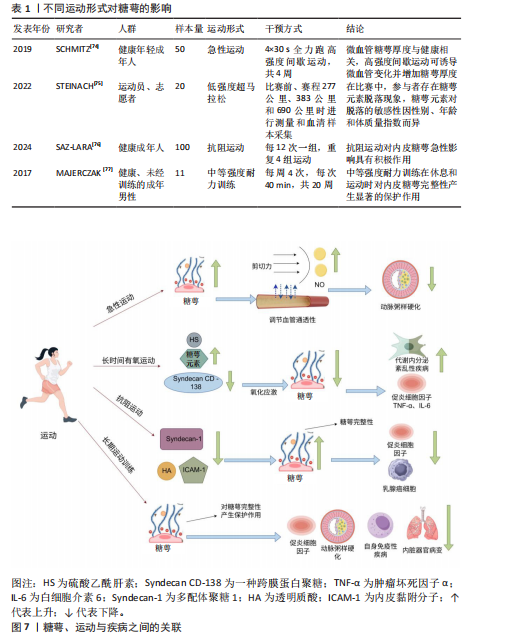
综上所述,巨噬细胞脂质通过调控核因子κB通路来保护糖萼,避免糖萼的降解损伤,以此来减少炎症因子的形成,防止由脂多糖诱导的巨噬细胞炎症。 抗凝血酶除通过结合硫酸乙酰肝素实现抗凝作用外,还对糖萼的结构稳定起着重要作用[56]。OKAMOTO等[57]和IBA等[58]研究证实,重组抗凝血酶可显著抑制促炎因子白细胞介素1β的产生,同时明显降低脓毒症相关死亡率。 2.3.4 糖萼与创伤手术的关联性 近期研究聚焦烧烫伤及外科手术等创伤性损伤与糖萼的关联机制。早在2018年,HALBGEBAUER等[59]就发现复合性创伤是导致糖萼生物学特性紊乱的一个重要因素。经过临床观察,复合性创伤与出血性休克并发时糖萼损伤程度显著加剧。接着,QI等[60]经过定量分析复合性创伤患者血清中的糖萼损伤脱落物,评估糖萼损伤与多系统创伤之间的关联。 在烧伤类病症中,WELLING等[61]发现患者血清中糖萼损伤脱落物含量上升与高温灼烧创伤密切相关。YANASE等[62]发现腹部外科手术患者血清糖萼损伤脱落物水呈现升高趋势,由此,部分学者提出用糖萼损伤脱落物作为评估创伤性损伤程度的一个指标。SUZUKI等[63]研究肝脏和胰腺手术患者体内糖萼的降解水平变化,术后糖萼结构性损伤并出现脱落,但通过白蛋白补充治疗可有效降低糖萼损伤程度。PASSOV等[64]对30例主动脉瓣置换术并发再灌注损伤病例的糖萼完整性进行定量分析,揭示心脏手术可诱发糖萼损伤病理过程。除此之外,SCHIEFER等[65]检测发现肾移植患者术后血清糖萼核心蛋白多配体聚糖1水平显著上升,说明器官移植手术会导致糖萼损伤。 综上所述,创伤性手术与糖萼完整性显著相关,可用糖萼损伤脱落物水平评估创伤性疾病的严重程度。 2.3.5 糖萼与其他疾病的关联性 关于糖萼损伤与内脏器官病变的关联性,INKINEN等[66]揭示糖萼降解产物会加速急性肾损伤的病理进程,ZHANG等[67]发现可通过靶向抑制糖萼降解改善急性肺损伤中的炎症、水肿等症状,表明糖萼的完整性对急性肺损伤非常重要。 有关代谢内分泌紊乱与感染性疾病对糖萼结构完整性的影响,KAUR等[68]通过检测糖尿病患者视网膜组织中糖萼主要成分的表达量与损伤脱落物含量发现,维持糖萼结构完整对于治疗糖尿病患者视网膜病变非常重要。LAM等[69]与DELAVERIS等[70]发现,登革热病毒与甲型流感病毒感染可诱导血浆中糖萼特异性降解产物的积聚,进而引发血管外渗漏并加剧病情;此外,糖萼的密度、糖基化修饰水平及结构特征通过调控病毒受体稳定性,直接影响宿主细胞对病毒的易感性。 糖萼完的整性与自身免疫疾病之间存在相关性。CLARKE[71]研究证实,系统性红斑狼疮与糖萼完整性损伤存在密切病理关联,靶向维持糖萼稳定性已成为该疾病治疗策略开发的新方向。最新研究揭示艾滋病病毒感染机制中糖萼的关键作用,SPILLINGS等[72]发现病毒表面糖蛋白与宿主细胞糖萼聚糖通过分子互补结合,提升病毒入侵效率,该互作机制为阻断艾滋病传播提供了潜在干预靶点。 综上所述,糖萼结构完整性与多种疾病进展存在显著关联,因此,靶向糖萼保护及其损伤后的修复有望成为相关疾病的新型治疗策略。 2.4 运动对糖萼分泌的影响 糖萼受运动持续时间、运动方式变化、运动强度大小等因素的影响。急性运动可以诱导微血管变化和增加糖萼厚度,而有氧运动中糖萼元素对脱落的敏感性因性别、年龄和体质量指数而异,抗阻运动对内皮糖萼的急性影响具有积极作用,长期运动训练可以起到保护糖萼的作用。 2.4.1 急性运动对糖萼的影响 急性运动为单次身体活动,即一次性非长期重复运动[73]。SCHMITZ等[74]研究评估4周高强度间歇性训练对健康年轻成年人微血管结构(包括内皮糖萼)及相关循环 miRNA的影响,发现高强度间歇性训练可能通过剪切应力和相关 miRNA发挥内皮糖萼保护作用,微血管糖萼厚度与健康相关,高强度间歇性训练可诱导微血管变化并增加糖萼厚度。通过身体锻炼如高强度间歇性训练可诱导微血管变化并增加糖萼厚度,这一过程能够促进血管保护、影响内皮机械传感与介导剪切应力依赖性一氧化氮产生、调节血管通透性,这对运动通过影响糖萼从而调控动脉粥样硬化和炎症的发病机制提供了重要思路。 2.4.2 有氧运动对糖萼的影响 有研究以参加育空北极超马拉松的13名运动员(6名女性,7名男性)和 7名志愿者(5名女性,2名男性)为研究对象,通过在比赛前、赛程277 公里、383公里和690公里时进行血清样本采集,分析了血清中糖萼参数(硫酸乙酰肝素、Syndecan CD-138等)的变化以及其他血清应激参数(皮质醇、C-反应蛋白、肌酸激酶、N末端 B型利钠肽原)[75]。结果发现在寒冷气候下的超长距离、低强度超马拉松比赛中,参与者存在糖萼元素脱落现象,糖萼元素对脱落的敏感性因性别、年龄和体质量指数而异,完成距离增加和N末端B型利钠肽原值升高与糖萼元素脱落增加相关。长时间有氧运动(如超马拉松)可能因氧化应激导致糖萼脱落,但糖萼脱落可能为适应性重组的前奏,促进后续修复,这些作为预防与治疗代谢内分泌紊乱性疾病、炎症等提供指导性意义;可能存在有利于糖萼稳定性的运动理想范围,糖萼的重组和脱落可能是耐力训练适应的必要步骤。 2.4.3 抗阻运动对糖萼的影响 一项研究招募100名18岁以上的受试者进行阻力训练,探究抗阻运动对内皮糖萼的急性影响,训练内容包括4组强度相当于100% 1次最大负重的卧推和腿部弯举,每12次重复一组,训练持续时间为30-60 min[76]。通过酶联免疫吸附实验对内皮糖萼成分的标志物进行评估,结果发现阻力训练改善健康人群的内皮糖萼功能及其成分动态,其中对蛋白多糖的调控作用尤为突出;同时,运动后糖萼相关分子(如多配体聚糖1、透明质酸)及内皮黏附分子(细胞间黏附分子1、血管细胞黏附分子1)的表达变化可减少炎症反应。 2.4.4 长期运动训练对糖萼的影响 MAJERCZAK等[77]评估了20周中等强度耐力训练对内皮糖萼层完整性的影响,以11名健康、未经训练的年轻男性为研究对象,发现耐力训练导致体能显著增加(P < 0.05),体现在乳酸阈值和最大摄氧量时的功率输出增加;训练导致糖萼损伤血液标志物(多配体聚糖1和硫酸肝素)的基础和运动末浓度降低(P < 0.05),训练后糖萼脱落的降低伴随着氧化应激的减弱,表现为异前列腺素基础血浆浓度的降低以及股外侧肌超氧化物歧化酶2蛋白含量的增加(P < 0.05),表明抗氧化防御能力的增强,相比之下,训练没有引起基础亚硝酸盐/硝酸盐血浆浓度的显著增加(P < 0.05),说明中等强度耐力训练在休息和运动过程中可能通过改善抗氧化防御对内皮糖萼完整性有明显保护作用,这可以作为预防动脉粥样硬化、炎症、自身免疫性疾病、内脏器官病变的非药物干预措施。不同运动形式对糖萼的影响见表1所示。糖萼、运动与疾病之间的关联如图7所示。"
| [1] 董佳慧,李慧敏,李南熹,等.糖萼与疾病的相关性及其降解机制研究进展[J].解放军医学杂志,2023,48(3):345-354. [2] GRIFFIN ME, SORUM AW, MILLER GM, et al. Sulfated glycans engage the Ang-Tie pathway to regulate vascular development. Nat Chem Biol. 2021;17(2):178-186. [3] 康红艳,刘佳佳,喻淼淼,等.血管内皮细胞糖萼的观测技术研究进展[J].生命科学,2016,28(9):1089-1099. [4] REITSMA S, SLAAF DW, VINK H. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454(3):345-359. [5] RICHTER RP, PAYNE GA, AMBALAVANAN N, et al. The endothelial glycocalyx in critical illness: a pediatric perspective. Matrix Biol Plus. 2022;14:100106. [6] ZHANG X, LIN L, HUANG H, et al. Chemoenzymatic synthesis of glycosaminoglycans. Acc Chem Res. 2019; 53(2):335-346. [7] SONG Y, ZHANG F, LINHARDT RJ. Glycosaminoglycans. Adv Exp Med Biol. 2021;1325:103-116. [8] MENDE M, BEDNAREK C, WAWRYSZYN M, et al. Chemical synthesis of glycosaminoglycans. Chem Rev. 2016;116(14):81938255. [9] KAMHI E, JOO EJ, DORDICK JS. Glycosaminoglycans in infectious disease. Biol Rev Camb Philos Soc. 2013;88(4):928-943. [10] ZIMMERMANN R, WERNER C, STERLING J. Exploring structure - property relationships of GAGs to tailor ECM-mimicking hydrogels. Polymers (Basel). 2018;10(12):1376. [11] SHENG A, CHEN Q, YU M. Coupling liquid chromatography and tandem mass spectrometry to electrophoresis for indepth analysis of glycosaminoglycan drugs: heparin and the multicomponent sulodexide. Anal Chem. 2020;93(3):14331442. [12] SODHI H, PANITCH A. Glycosaminoglycans in tissue engineering: a review. Biomolecules. 2021;11(1):29. [13] PIPERIGKOU Z, TZAFERI K, MAKROKANIS G, et al. The microRNA-cell surface proteoglycan axis in cancer progression. Am J Physiol Cell Physiol. 2022;322(5):825-832. [14] JIN J, FANG F, GAO W, et al. The structure and function of the glycocalyx and its connection with blood-brain barrier. Front Cell Neurosci. 2021;15:739699. [15] FU L, KIM HN, STERLING JD, et al. The role of the cell surface glycocalyx in drug delivery to and through the endothelium. Adv Drug Deliv Rev. 2022;184:114195. [16] COELHO-SANTOS V, SHIH AY. Postnatal development of cerebrovascular structure and the neurogliovascular unit. Wiley Interdiscip Rev Dev Biol. 2020;9(2):e363. [17] MASOLA V, GRECO N, GAMBARO G, et al. Heparanase as active player in endothelial glycocalyx remodeling. Matrix Biol Plus. 2022;13:100097. [18] ZHANG C, GUO F, CHANG M, et al. Exosome-delivered syndecan-1 rescues acute lung injury via a FAK/ p190RhoGAP/RhoA/ROCK/NF-kappaB signaling axis and glycocalyx enhancement. Exp Cell Res. 2019;384(1):111596. [19] AFRATIS N, GIALELI C, NIKITOVIC D. Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 2012; 279(7):1177-1197. [20] COSGUN ZC, FELS B, KUSCHE-VIHROG K. Nanomechanics of the endothelial glycocalyx: from structure to function Am J Pathol. 2020;190(4):732-741. [21] MAJERCZAK J, DUDA K, CHLOPICKI S, et al. Endothelial glycocalyx integrity is preserved in young, healthy men during a single bout of strenuous physical exercise. Physiol Res. 2016;65(2):281-291. [22] SCHMITZ B, BREULMANN FL, JUBRAN B, et al. A three-step approach identifies novel shear stress-sensitive endothelial microRNAs involved in vasculoprotective effects of high-intensity interval training (HIIT). Oncotarget. 2019;10(38):3625-3640. [23] BUFFONE A, WEAVER VM. Don’t sugarcoat it: How glycocalyx composition influences cancer progression. J Cell Biol. 2020;219(1):e201910070. [24] KADOYA H, YU N, SCHIESSL IM, et al. Essential role and therapeutic targeting of the glomerular endothelial glycocalyx in lupus nephritis. JCI Insight. 2020;5(19): e131252. [25] 林源希,李真玉.糖萼在脓毒症血管内皮损伤中的变化及其修复策略研究进展[J].解放军医学杂志,2022,47(10):1049-1056. [26] CHELAZZI C, VILLA G, MANCINELLI P, et al. Glycocalyx and sepsisinduced alterations in vascular permeability. Crit Care. 2015;19: 26. [27] ALPHONSUS CS, RODSETH RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia. 2014;69(7): 777-784. [28] 马壮,修光辉,熊伟,等.血管内皮糖萼的研究进展[J].广东医学,2019,40(14): 2113-2116. [29] KORAKAS E, IKONOMIDIS I, MARKAKIS K, et al.The Endothelial Glycocalyx as a Key Mediator of Albumin Handling and the Development of Diabetic Nephropathy. Curr Vase Pharmacol. 2020;18(6):619-631. [30] HU Z, CANO I, D’AMORE PA. Update on the role of the endothelial glycocalyx in angiogenesis and vascular inflammation. Front Cell Dev Biol. 2021;9:734276. [31] 张梓航,高朵岚,戴鑫源,等.衰老引发的糖萼损伤与血管功能障碍[J].医用生物力学,2024,39(2):368-374. [32] TONG W, DUAN Y, YANG R, et al. Foam cell-derived CXCL14 muti-functionally promotes atherogenesis and is a potent therapeutic target in atherosclerosis. J Cardiovasc Transl Res. 2020;13(2):215-224. [33] KOZAR RA, PATI S. Syndecan-1 restitution by plasma after hemorrhagic shock. J Trauma Acute Care Surg. 2015;78(6 Suppl 1): S83-S86. [34] KIM YH, NIJST P, KIEFER K, et al. Endothelial glycocalyx as biomarker for cardiovascular diseases: mechanistic and clinical implications. Curr Heart Fail Rep. 2017;14(2):117-126. [35] HAJIBABAIE F, KOUHPAYEH S, MIRIAN M, et al. MicroRNAs as the actors in the atherosclerosis scenario. J Physiol Biochem. 2020;76(1):1-12. [36] MIRANDA CH, DE CARVALHO BM, SCHMIDT A, et al. Evaluation of the endothelial glycocalyx damage in patients with acute coronary syndrome. Atherosclerosis. 2016; 247:184-188. [37] NEMOTO T, MINAMI Y, YAMAOKA-TOJO M, et al. Endothelial glycocalyx and severity and vulnerability of coronary plaque in patients with coronary artery disease. Atherosclerosis. 2020;302:1-7. [38] CANCEL LM, EBONG EE, MENSAH S, et al. Endothelial glycocalyx, apoptosis and inflammation in an atherosclerotic mouse model. Atherosclerosis. 2016;252:136-146. [39] BUTLER PJ, BHATNAGAR A. Mechanobiology of the abluminal glycocalyx. Biorheology. 2019;56(2-3):101-112. [40] HOJMAN P, DETHLEFSEN C, BRANDT C, et al. Exercise-induced musclederived cytokines inhibit mammary cancer cell growth. Am J Physiol Endocrinol Metab. 2011;301(3):E504-E510. [41] VALLA S, HASSAN N, VITALE DL, et al. Syndecan-1 depletion has a differential impact on hyaluronic acid metabolism and tumor cell behavior in luminal and triple-negative breast cancer cells. Int J Mol Sci. 2021;22(11):5874. [42] MORAN H, CANCEL LM, HUANG P, et al. Glycocalyx mechanotransduction mechanisms are involved in renal cancer metastasis. Matrix Biol Plus. 2022;13: 100100. [43] TONDEPU C, KARUMBAIAH L. Glycomaterials to investigate the functional role of aberrant glycosylation in glioblastoma. Adv Healthc Mater. 2022;11(4):e2101956. [44] OLIVEIRA T, ZHANG M, JOO EJ, et al. Glycoproteome remodeling in MLL-rearranged B-cell precursor acute lymphoblastic leukemia. Theranostics. 2021;11(19):9519-9537. [45] BECKER BF, CHAPPELL D, BRUEGGER D, et al. TheraPeutic strategies targeting the endothelial glycocalyx:acute deficits,but great potential.Cardiovasc Res. 2010; 87(2):300-310. [46] CORREA PL, ISHITANI LH, ABREU DX, et al. The importance of surveillance in cases of and mortality from the COVID-19 epidemic in Belo Horizonte, Brazil, 2020. Rev Bras Epidemiol. 2020;23:e200061. [47] CHEN F, WANG XD, ZHU KK, et al. Investigation of the psychological status of suspected pati ents during the Coronavirus disease 2019 epidemic. Medicine (Baltimore). 2020;99(38): e22260. [48] LAMBADIARI V, MITRAKOU A, KOUNTOURI A, et al. Association of COVID-19 with impaired endothelial glycocalyx, vascular function and myocardial deformation 4 months after infection. Eur J Heart Fail. 2021;23(11):1916-1926. [49] QUEISSER KA, MELLEMA RA, MIDDLETON EA, et al. COVID-19 generates hyaluronan fragments that directly induce endothelial barrier dysfunction. JCI Insight. 2021;6(17): e147472. [50] PREEZ HN, ALDOUS C, HAYDEN MR, et al. Pathogenesis of COVID-19 described through the lens of an undersulfated and degraded epithelial and endothelial glycocalyx. FASEB J. 2022;36(1):e22052. [51] MASOLA V, ZAZA G, ARDUINI A, et al. Endothelial glycocalyx as a regulator of fibrotic processes. Int J Mol Sci. 2021; 22(6):2996. [52] SONG JW, ZULLO J, LIPPHARDT M. Endothelial glycocalyx-the battleground for complications of sepsis and kidney injury. Nephrol Dial Transplant. 2018;33(2):203-211. [53] VREYS V, DAVID G. Mammalian heparanase: what is the message? J Cell Mol Med. 2007;11(3): 427-452. [54] CHEN S, HE Y, HU ZW, et al. Heparanase mediates intestinal inflammation and injury in a mouse model of sepsis. J Histochem Cytochem. 2017;65(4):241-249. [55] LI H, HAO Y, YANG LL, et al . MCTR 1 alleviates lipopolysaccharide-induced acute lung injury by protecting lung endothelial glycocalyx. J Cell Physiol. 2020; 235(10):7283-7294. [56] MYERS GJ, WEGNER J. Endothelial glycocalyx and cardiopulmonary bypass. J Extra Corpor Technol. 2017;49(3):174-181. [57] OKAMOTO H, MURAKI I, OKADA H, et al. Re comb inant antithrombin attenuates acute respiratory distress syndrome in experimental endotoxemia. Am J Pathol. 2021;191(9):1526-1536. [58] IBA T, LEVY JH, AIHARA K, et al. Newly developed recombinant antithrombin protects the endothelial glycocalyx in an endotoxin-induced rat model of sepsis. Int J Mol Sci. 2020; 22(1):176. [59] HALBGEBAUER R, BRAUN CK, DENK S, et al. Hemorrhagic shock drives glycocalyx, barrier and organ dysfunction early after polytrauma. J Crit Care. 2018;44:229-237. [60] QI F, ZHOU H, GU P, et al. Endothelial glycocalyx degradation is associated with early organ impairment in poly trauma patients. BMC Emerg Med. 2021;21(1):52. [61] WELLING H, HENRIKSEN HH, GONZALEZ-RODRIGUEZ ER, et al. Endothelial glycocalyx shedding in patients with burns. Burns. 2020;46(2):386-393. [62] YANASE F, NAORUNGROJ T, BELLOMO R. Glycocalyx damage biomarkers in healthy controls, abdominal surgery, and sepsis: a scoping review. Biomarkers. 2020;25(6): 425-435. [63] SUZUKI T, KOYAMA K. Open randomized trial of the effects of 6% hydroxyethyl starch 130/0.4/9 and 5% albumin on safety profile, volume efficacy, and glycocalyx degradation in hepatic and pancreatic surgery. J Anesth. 2020;34(6): 912-923. [64] PASSOV A, SCHRAMKO A, SALMINEN US, et al. Endothelial glycocalyx during early reperfusion in patients undergoing cardiac surgery. PLoS One. 2021;16(5):e0251747. [65] SCHIEFER J, FAYBIK P, KOCH S, et al. Glycocalyx damage within human liver grafts correlates with graft injury and postoperative graft function after orthotopic liver transplantation. Transplantation. 2020;104(1):72-78. [66] INKINEN N, PETTILA V, LAKKISTO P, et al. Association of endothelial and glycocalyx injury biomarkers with fluid administration,development of acute kidney injury, and 90-day mortality: data from the FINNAKI observational study. Ann Intensive Care. 2019;9(1):103. [67] ZHANG C, GUO F, CHANG M, et al. Exosome-delivered syndecan-1 rescues acute lung injury via a FAK/p190RhoGAP/RhoA/ ROCK/NF-κB signaling axis and glycocalyx enhancement. Exp Cell Res. 2019;384(1):111596. [68] KAUR G, ROGERS J, RASHDAN NA, et al. Hyperglycemia-induced effects on glycocalyx components in the retina. Exp Eye Res. 2021;213:108846. [69] LAM PK, MCBRIDE A, LE DT, et al. Visual and biochemical evidence of glycocalyx disruption in human dengue infection, and association with plasma leakage severity. Front Med. 2020;7:545813. [70] DELAVERIS CS, WEBSTER ER, BANIK SM, et al. Membrane-tethered mucin-like polypeptides sterically inhibit binding and slow fusion kinetics of influenza A virus. Proc Natl Acad Sci U S A. 2020;117(23): 12643-12650. [71] CLARKE J. The glycocalyx presents a sweet new target in lupus nephritis. Nat Rev Rheumatol. 2020;16(11):604. [72] SPILLINGS BL, DAY CJ, GARCIA-MINAMBRES A, et al. Host glycocalyx captures HIV proximal to the cell surface via oligomannoseGlcNAc glycan-glycan interactions to support viral entry. Cell Rep. 2022;38(5):110296. [73] BASSO JC, SUZUKI WA. The Effects of Acute Exercise on Mood, Cognition, Neurophysiology, and Neurochemical Pathways: A Review. Brain Plast. 2017;2(2): 127-152. [74] SCHMITZ B, NIEHUES H, LENDERS M, et al. Effects of high-intensity interval training on microvascular glycocalyx and associated microRNAs. American journal of physiology. Am J Physiol Heart Circ Physiol. 2019;316(6):H1538-H1551. [75] STEINACH M, BIERE K, COKER RH, et al. Influences on glycocalyx shedding during the Yukon Arctic Ultra: the longest and the coldest ultramarathon. J Appl Physiol. 2022;133(5):1119-1135. [76] SAZ-LARA A, CAVERO-REDONDO I, DEL SAZ-LARA A, et al. The acute effect of exercise on the endothelial glycocalyx in healthy adults: A systematic review and meta-analysis. Eur J Clin Invest. 2024; 54(9):e14240. [77] MAJERCZAK J, GRANDYS M, DUDA K, et al. Moderate-intensity endurance training improves endothelial glycocalyx layer integrity in healthy young men. Exp Physiol. 2017;102(1):70-85. [78] DOGNÉ S, RATH G, JOURET F, et al. Hyaluronidase 1 Deficiency Preserves Endothelial Function and Glycocalyx Integrity in Early Streptozotocin-Induced Diabetes. Diabetes. 2016;65(9):2742-2753. [79] AABASSI Z, ARMALY Z, HEYMAN SN. Glycocalyx Degradation in Ischemia-Reperfusion Injury. Am J Pathol. 2020; 190(4):752-767. [80] ARUNKUMAR N, VU DC, KHAN S. Diagnosis of mucopolysaccharidoses and mucolipidosis by assaying multiplex enzymes and glycosaminoglycans. Diagnostics. 2021; 11(8):1347. [81] SAPP RM, EVANS WS, EAGAN LE, et al. The effects of moderate and high-intensity exercise on circulating markers of endothelial integrity and activation in young, healthy men. J Appl Physiol (1985). 2019;127(5):1245-1256. |
| [1] | Zhang Qingtong, Chen Leqin, Liu Chang, Chen Yuting, Guo Ruiwu. Neuromechanism of the endocannabinoid system in regulating exercise motivation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(在线): 1-11. |
| [2] | Zhang Xianxu, Ma Zhong, Liu Xin, Huang Lei, Shen Wenxiang, Luo Zhiqiang . Lumbar fusion combined with unilateral fixation for lumbar degenerative diseases: biomechanics, technical evolution, and clinical applications [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2334-2342. |
| [3] | Liu Jinlong, Abuduwupuer·Haibier, Bai Zhen, Su Danyang, Miao Xin, Li Fei, Yang Xiaopeng. Efficacy of different nonsurgical treatments for adolescent idiopathic scoliosis: a systematic review and network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2370-2379. |
| [4] | Wen Fayan, Li Yan, Qiang Tianming, Yang Chen, Shen Linming, Li Yadong, Liu Yongming. Unilateral biportal endoscopic technology for treatment of lumbar degenerative diseases: global research status and changing trends [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2380-2390. |
| [5] | Fu Lyupeng, Yu Peng, Liang Guoyan, Chang Yunbing. Electroactive materials applied in spinal surgery [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2113-2123. |
| [6] | Yang Xuetao, Zhu Menghan, Zhang Chenxi, Sun Yimin, Ye Ling. Applications and limitations of antioxidant nanomaterials in oral cavity [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2044-2053. |
| [7] | Pan Dong, Yang Jialing, Tian Wei, Wang Dongji, Zhu Zheng, Ma Wenchao, Liu Na, Fu Changxi. Resistance exercise activates skeletal muscle satellite cells in aged rats: role of adiponectin receptor 1 pathway [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1736-1746. |
| [8] | Chen Yulin, He Yingying, Hu Kai, Chen Zhifan, Nie Sha Meng Yanhui, Li Runzhen, Zhang Xiaoduo , Li Yuxi, Tang Yaoping. Effect and mechanism of exosome-like vesicles derived from Trichosanthes kirilowii Maxim. in preventing and treating atherosclerosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1768-1781. |
| [9] | Tao Daiju, Su Haiyu, Wang Yuqi, Shen Zhiqiang, He Bo . Construction and identification of stable PC12 cell lines with high/low expression of miR-122-5p [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1790-1799. |
| [10] | Liu Anting, Lu Jiangtao, Zhang Wenjie, He Ling, Tang Zongsheng, Chen Xiaoling. Regulation of AMP-activated protein kinase by platelet lysate inhibits cadmium-induced neuronal apoptosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1800-1807. |
| [11] | Cao Yong, Teng Hongliang, Tai Pengfei, Li Junda, Zhu Tengqi, Li Zhaojin. Interactions between cytokines and satellite cells in muscle regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1808-1817. |
| [12] | Fan Yongjing, Jin Wulong, Bai Haoyu, Ma Ping, Wang Shu. Role and mechanism of stem cells from human exfoliated deciduous teeth in tissue regeneration and disease treatment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1850-1857. |
| [13] | Lai Jiaming, , Song Yuling, Chen Zixi, Wei Jinghuan, Cai Hao, , Li Guoquan, . Screening of diagnostic markers for endothelial cell Senescence in mice with radiation-induced heart disease and analysis of immune infiltration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1450-1463. |
| [14] | Hou Chaowen, Li Zhaojin, Kong Jianda, Zhang Shuli. Main physiological changes in skeletal muscle aging and the multimechanism regulatory role of exercise [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1464-1475. |
| [15] | Sun Yaotian, Xu Kai, Wang Peiyun. Potential mechanisms by which exercise regulates iron metabolism in immune inflammatory diseases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1486-1498. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||