Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (7): 1790-1799.doi: 10.12307/2026.082
Previous Articles Next Articles
Construction and identification of stable PC12 cell lines with high/low expression of miR-122-5p
Tao Daiju, Su Haiyu, Wang Yuqi, Shen Zhiqiang, He Bo
- School of Pharmaceutical Science & Yunnan Key Laboratory of Pharmacology for Natural Products, Kunming Medical University, Kunming 650500, Yunnan Province, China
-
Received:2024-11-12Revised:2025-05-21Accepted:2025-06-20Online:2026-03-08Published:2025-08-20 -
Contact:Shen Zhiqiang, PhD, Professor, School of Pharmaceutical Science & Yunnan Key Laboratory of Pharmacology for Natural Products, Kunming Medical University, Kunming 650500, Yunnan Province, China He Bo, PhD, Professor, School of Pharmaceutical Science & Yunnan Key Laboratory of Pharmacology for Natural Products, Kunming Medical University, Kunming 650500, Yunnan Province, China He Bo, PhD, Professor, School of Pharmaceutical Science & Yunnan Key Laboratory of Pharmacology for Natural Products, Kunming Medical University, Kunming 650500, Yunnan Province, China -
About author:Tao Daiju, Doctoral candidate, School of Pharmaceutical Science & Yunnan Key Laboratory of Pharmacology for Natural Products, Kunming Medical University, Kunming 650500, Yunnan Province, China -
Supported by:National Natural Science Foundation of China, No. 82260271 (to HB); Science and Technology Project of Science and Technology Department of Yunnan Province, No. 202001AY070001-157 (to SZQ)
CLC Number:
Cite this article
Tao Daiju, Su Haiyu, Wang Yuqi, Shen Zhiqiang, He Bo . Construction and identification of stable PC12 cell lines with high/low expression of miR-122-5p[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1790-1799.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
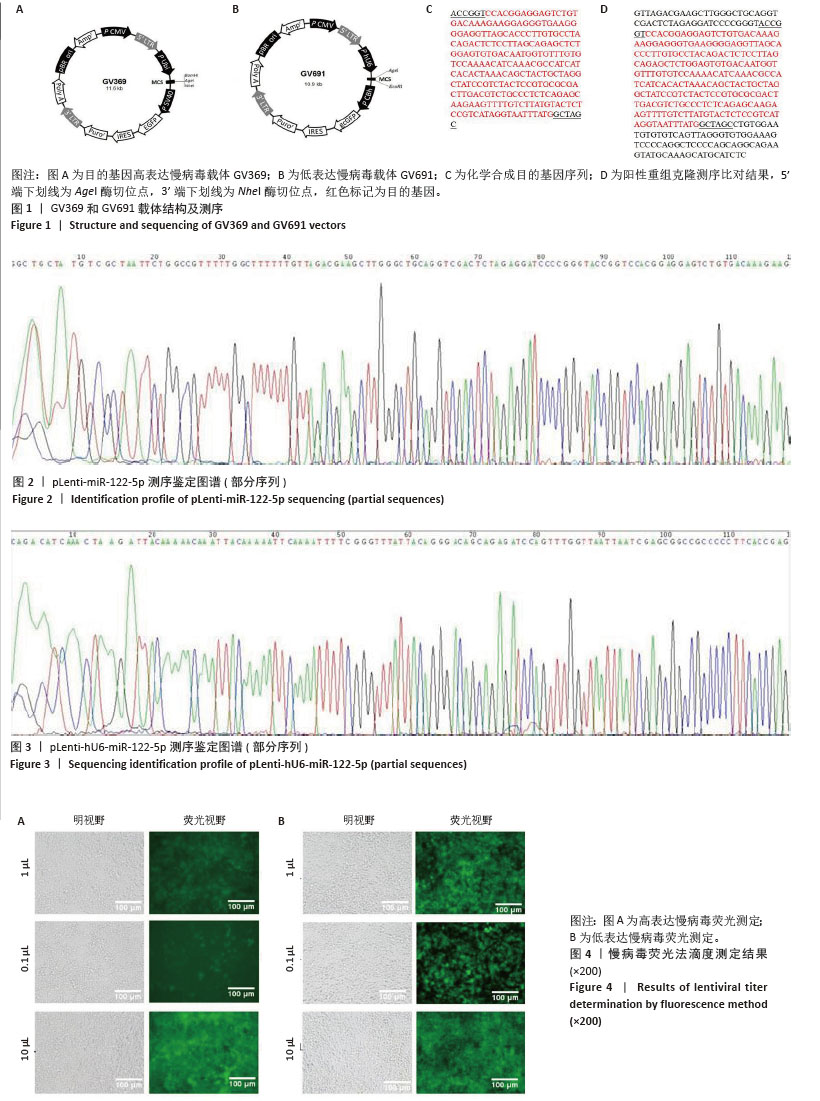
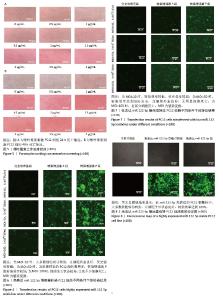
2.1 慢病毒载体构建及质粒鉴定 如图1所示,此次研究成功构建了GV369和GV691两种慢病毒载体,分别用于miR-122-5p的高表达和低表达(图1A,B)。经37 ℃培养18 h后,筛选得到的阳性单克隆菌落进行测序分析,所得序列与目标基因的反向互补序列比对结果显示完全匹配(图1D)。如图1C所示,合成的目的基因序列经测序验证证实已准确插入GV369载体(图2,3),测序结果与预期设计序列完全一致。 2.2 慢病毒的包装及滴度测定 进一步对慢病毒进行包装及滴度测定,结果显示,miR-122-5p 高表达慢病毒滴度为4×108 TU/mL,miR-122-5p低表达慢病毒滴度为1×109 TU/mL(图4)。"
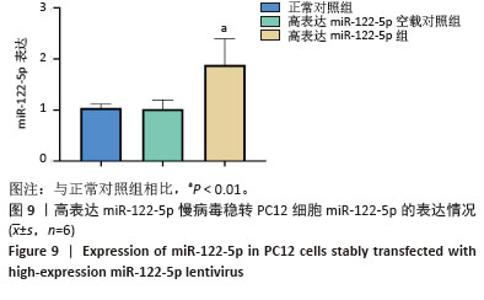
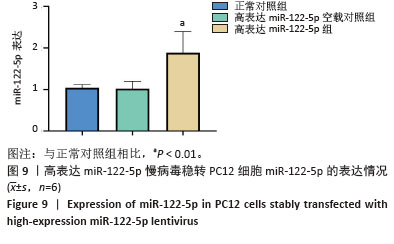
2.3 嘌呤霉素处理PC12细胞后的死亡情况 在将PC12细胞铺板于24孔板并培养24 h后更换各培养孔的培养基,使其含有不同嘌呤霉素质量浓度。培养24 h后在显微镜下观察到,随着嘌呤霉素质量浓度的增加,PC12细胞死亡情况愈加明显,当质量浓度达到4 μg/mL及以上时,基本无细胞存活(图5A)。此外,随着培养时间的延长,即使嘌呤浓度仅为3.5 μg/mL,也无细胞存活(图5B)。 2.4 高/低表达miR-122-5p慢病毒最佳转染条件 将生长状态良好的PC12细胞进行转染,根据不同条件加入相应的转染试剂和慢病毒,并在荧光显微镜下观察细胞转染情况并拍照记录保存。实验结果表明,在MOI=10条件下,高表达miR-122-5p慢病毒转染PC12细胞效果良好,转染增强液P组表现出最优的转染效率(荧光强度显著)和细胞存活率,显著优于完全培养基组和转染增强液A组(图6)。随着MOI值增加至50,各组转染效率均有提升,其中转染增强液P组仍保持最佳转染效果;但当MOI升至100时,细胞存活率明显下降。对于低表达miR-122-5p慢病毒转染,MOI=10时转染效率欠佳(荧光信号较弱),MOI=50时转染效率可达90%且细胞状态良好,而MOI=100则导致部分细胞死亡(图7)。综合细胞状态、病毒用量和荧光强度等因素最终确定,当MOI=10且使用转染增强液HiTransG P进行高表达miR-122-5p慢病毒转染时转染效果最佳;在MOI=50使用转染增强液HiTransG P低表达miR-122-5p 慢病毒转染时转染效果最佳。 2.5 高表达miR-122-5p慢病毒稳转PC12细胞株miR-122-5p的表达量 将转染增强液HiTransG P及MOI=10高表达miR-122-5p的慢病毒对PC12细胞进行转染,连续培养72 h,然后添加3.5 μg/mL嘌呤霉素进行筛选,培养四五代后获得miR-122-5p稳定高表达的PC12细胞株。荧光显微镜结果显示,在miR-122-5p高表达的PC12细胞株中,大多数细胞成功转染,且细胞生长状态良好,转染效率达到100%,见图8。通过提取总RNA进行qPCR定量检测,结果显示,与正常对照组相比,高表达miR-122-5p组的miR-122-5p表达量有显著升高,差异有显著性意义(P < 0.01),而高表达miR-122-5p空载对照组的miR-122-5p表达量未发生明显变化,见图9。"
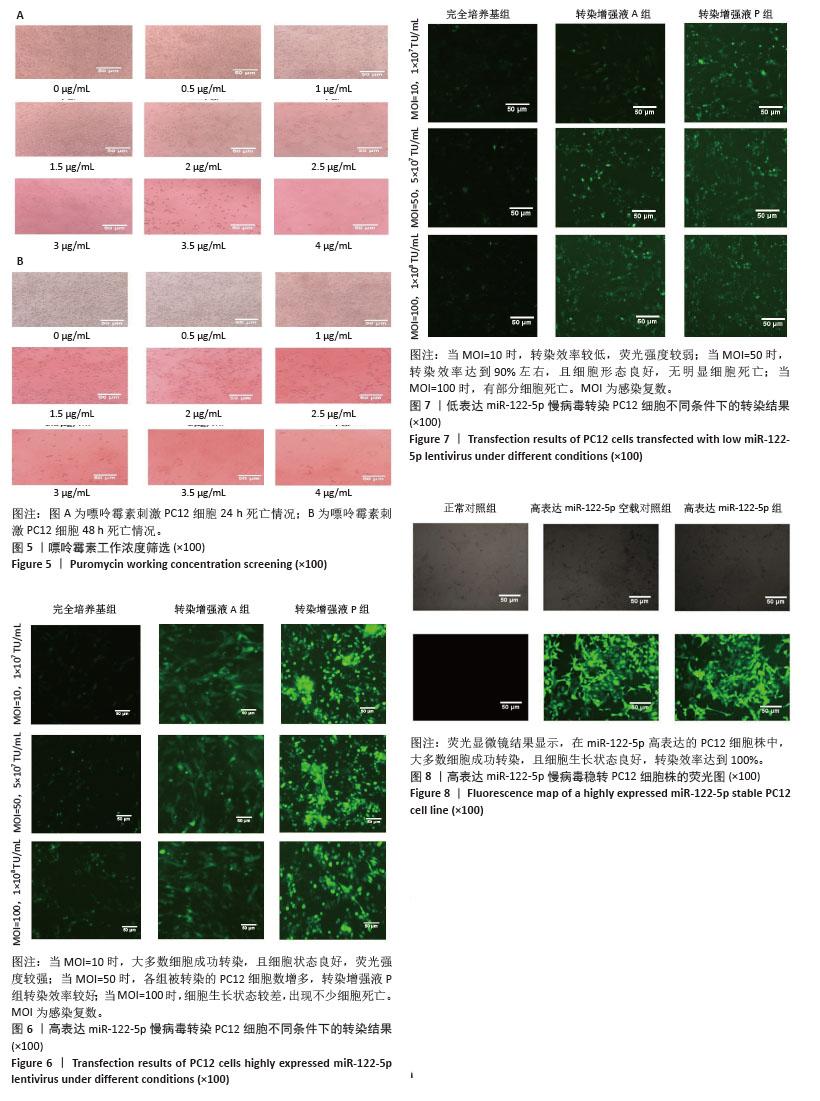
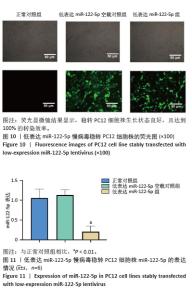
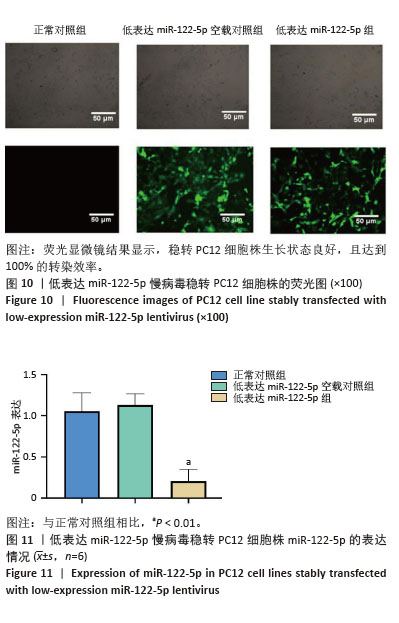
2.6 低表达miR-122-5p慢病毒转染PC12细胞株miR-122-5p的表达量 将含转染增强液HiTransG P以及MOI=50的低表达慢病毒加入完全培养基中,与PC12细胞共培养72 h,随后使用3.5 μg/mL嘌呤霉素筛选获得了稳定低表达PC12细胞株。荧光显微镜结果显示,稳转PC12细胞株生长状态良好,且达到100%的转染效率(图10)。进一步对该细胞株提取总RNA并进行RT-qPCR分析,结果表明,与正常对照组比较,低表达miR-122-5p组的miR-122-5p表达量有显著降低,差异有显著性意义(P < 0.01), 而低表达miR-122-5p空载对照组的miR-122-5p表达量未出现明显变化(图11)。 "
| [1] FU G, BRKIĆ J, HAYDER H, et al. MicroRNAs in Human Placental Development and Pregnancy Complications. Int J Mol Sci. 2013;14(3): 5519-5544. [2] CHO JR, LEE CY, LEE J, et al. MicroRNA-761 inhibits Angiotensin II-induced vascular smooth muscle cell proliferation and migration by targeting mammalian target of rapamycin.Clin Hemorheol Micro. 2015;63(1):45-56. [3] LIN J, ZHAN R. [Advance of studies on role of miRNA in hematopoietic regulation and myeloproliferative neoplasms] Chin Assoc Pathophysiol. 2011;19:1071-1074. [4] LEE Y, AHN C, HAN J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425(6956):415-419. [5] BARTEL DP. Metazoan MicroRNAs. Cell. 2018;173(1):20-51. [6] SAIYED AN, VASAVADA AR, JOHAR SRK. Recent trends in miRNA therapeutics and the application of plant miRNA for prevention and treatment of human diseases.Futur J Pharm Sci. 2022;8(1):24. [7] CHIPMAN LB, PASQUINELLI AE. miRNA Targeting: Growing beyond the Seed. Trends Genet. 2019;35(3):215-222. [8] AZIMI M, TOTONCHI M, EBRAHIMI M. Determining The Role of MicroRNAs in Self-Renewal, Metastasis and Resistance to Drugs in Human Gastric Cancer Based on Data Mining Approaches: A Systematic Review. Cell J. 2022;24(1):1-6. [9] GOLHANI V, RAY SK, MUKHERJEE S. Role of MicroRNAs and Long Non-Coding RNAs in Regulating Angiogenesis in Human Breast Cancer: A Molecular Medicine Perspective. Curr Mol Med. 2022;22(10):882-893. [10] MA L, HUO Y, TANG Q, et al. Human Breast Milk Exosomal miRNAs are Influenced by Premature Delivery and Affect Neurodevelopment. Mol Nutr Food Res. 2024;68(9):e2300113. [11] ASAKIYA C, ZHU L, YUHAN J, et al. Current progress of miRNA-derivative nucleotide drugs: modifications, delivery systems, applications. Expert Opin Drug Del. 2022;19(4):435-450. [12] MAGED AM, DEEB WS, EL AMIR A, et al. Diagnostic accuracy of serum miR-122 and miR-199a in women with endometriosis.Int J Gynecol Obstet. 2018;141(1):14-19. [13] ZHAN G, JIANG H, YANG R, et al. miR-122 and miR-197 expressions in hepatic carcinoma patients before and after chemotherapy and their effect on patient prognosis. Am J Transl Res. 2021;13(6):6731-6737. [14] ZHANG K, XU X, HU L. Sevoflurane attenuates hepatic ischemia reperfusion injury by the miR-122/Nrf2 pathway.Ann Transl Med. 2022;10(6):350. [15] WANG L, CHEN H. Correlation between serum miR-122 and myocardial damage and ventricular function in patients with essential hypertension. J Thorac Dis. 2021;13(8):4999-5006. [16] JAMPOKA K, MUANGPAISARN P, KHONGNOMNAN K, et al. Serum miR-29a and miR-122 as Potential Biomarkers for Non-Alcoholic Fatty Liver Disease (NAFLD). Microrna. 2018;7(3):215-222. [17] WU X, DU X, YANG Y, et al. Inhibition of miR-122 reduced atherosclerotic lesion formation by regulating NPAS3-mediated endothelial to mesenchymal transition.Life Sci. 2021;265:118816. [18] CHEN Z, FU S, SHAN Y, et al. Circ_0001047 inhibits prostate cancer progression and enhances abiraterone sensitivity via miR-122-5p/FKBP5/PHLPP1/AKT axis in vitro. Discov Oncol. 2024;15(1):569. [19] VOKACOVA K, LANDECKA A, SELVI S, et al. Plasma miR-122-5p and miR-142-5p and their role in chemoresistance of colon cancer patients. Mutagenesis. 2025;40(1):80-86. [20] ZHANG J, LI K, GAO L, et al. Glucose metabolism disorder related to follicular fluid exosomal miR-122-5p in cumulus cells of endometriosis patients. Reproduction. 2024;168(4):e240028. [21] LIU F, LIU B, XU S, et al. MicroRNA-122 protects against interferon-α-induced hepatic inflammatory response via the Janus kinase-signal transducer and activator of transcription pathway. Endocr J. 2025;72(1):53-67. [22] SONG C, LI S, MAI Y, et al. Dysregulated expression of miR-140 and miR-122 compromised microglial chemotaxis and led to reduced restriction of AD pathology. J Neuroinflammation. 2024;21(1):167. [23] LIN J, ZHENG X. Salvianolate Blocks Apoptosis During Myocardial Infarction by Down Regulating miR-122-5p. Curr Neurovasc Res. 2017; 14(4): 323-329. [24] MANKHONG S, KIM S, MOON S, et al. Circulating micro-RNAs Differentially Expressed in Korean Alzheimer’s Patients With Brain Aβ Accumulation Activate Amyloidogenesis. J Gerontol A-Biol. 2023;78(2): 292-303. [25] 王蒙蒙,王怡,林平. miR-122与急性缺血性脑卒中的相关性[J].中国老年学杂志,2022,42(5):1053-1055. [26] 黄蔚文, 赵岳峰. 重型颅脑损伤患者外周血miR-122-5p表达水平变化及其对近期预后的预测价值[J].卒中与神经疾病,2021,28(1): 88-91. [27] LEWIS BP, BURGE CB, BARTEL DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15-20. [28] PENG Y, CROCE CM. The role of MicroRNAs in human cancer. Signal Transduct Tar. 2016;1:15004. [29] CAN U, MARZIOGLU E, AKDU S. Some miRNA expressions and their targets in ischemic stroke. Nucleos Nucleot Nucl. 2022;41(11): 1224-1262. [30] YANG F, NING Z, MA L, et al. Exosomal miRNAs and miRNA dysregulation in cancer-associated fibroblasts. Mol Cancer. 2017;16(1): 148. [31] BAEK J, KANG S, MIN H. MicroRNA-targeting therapeutics for hepatitis C. Arch Pharm Res. 2014;37(3):299-305. [32] KHOODORUTH MAS, KHOODORUTH WNC, UROOS M, et al. Diagnostic and mechanistic roles of MicroRNAs in neurodevelopmental & neurodegenerative disorders. Neurobiol Dis. 2024;202:106717. [33] LIU T, WU H, LI J, et al. Unraveling the Bone-Brain Axis: A New Frontier in Parkinson’s Disease Research. Int J Mol Sci. 2024;25(23):12842. [34] SAWANT H, SUN B, MCGRADY E, et al. Role of miRNAs in neurovascular injury and repair. J Cerebr Blood F Met. 2024;44(10):1693-1708. [35] HINTERMAYER MA, JUŹWIK CA, MORQUETTE B, et al. A miR-383-5p Signaling Hub Coordinates the Axon Regeneration Response to Inflammation. J Neurosci. 2024;44(44):e1822232024. [36] 郭占非,吴洁,杨学华.miR-122-5p对类风湿关节炎滑膜细胞增殖与凋亡的影响及其机制[J].青岛大学学报(医学版),2022,58(2): 289-293. [37] VIBO R, JÕGI K, REMM A, et al. Different expression patterns of inflammation-related genes and serum microRNAs in young-onset ischemic stroke. Sci Rep. 2024;14(1):23845. [38] 罗凯, 段华彬, 罗明鼎.瑞舒伐他汀抑制miR-122-5p减轻LPS诱导的神经细胞损伤[J].河北医药,2022,44(5):659-663. [39] LV B, CHENG X, SHARP FR, et al. MicroRNA-122 Mimic Improves Stroke Outcomes and Indirectly Inhibits NOS2 After Middle Cerebral Artery Occlusion in Rats. Front Neurosci. 2018;12:767. [40] GUO D, MA J, LI T, et al. Up-regulation of miR-122 protects against neuronal cell death in ischemic stroke through the heat shock protein 70-dependent NF-κB pathway by targeting FOXO3. Exp Cell Res. 2018; 369(1):34-42. [41] MARTIN TF, GRISHANIN RN. PC12 cells as a model for studies of regulated secretion in neuronal and endocrine cells.Method Cell Biol. 2003;71: 267-286. [42] XIE D, DENG T, ZHAI Z, et al. The cellular model for Alzheimer’s disease research: PC12 cells.Front Mol Neurosci. 2022;15:1016559. [43] SAVENKOVA DA, MAKAROVA AA, SHALIK IK, et al. miRNA Pathway Alteration in Response to Non-Coding RNA Delivery in Viral Vector-Based Gene Therapy. Int J Mol Sci. 2022;23(23):14954. [44] SKUKAN L, BREZAK M, ISTER R, et al. Lentivirus- or AAV-mediated gene therapy interventions in ischemic stroke: A systematic review of preclinical in vivo studies. J Cerebr Blood F Met. 2022;42(2):219-236. |
| [1] | Wang Zheng, Cheng Ji, Yu Jinlong, Liu Wenhong, Wang Zhaohong, Zhou Luxing. Progress and future perspectives on the application of hydrogel materials in stroke therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2081-2090. |
| [2] | Wang Zheng, Cheng Ji, Yu Jinlong, Liu Wenhong, Wang Zhaohong, Zhou Luxing. Progress and future perspectives on the application of hydrogel materials in stroke therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-10. |
| [3] | Yu Jingbang, Wu Yayun. Regulatory effect of non-coding RNA in pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1659-1666. |
| [4] | Guo Daxin, Fan Susu, Zhu Zhendong, Hou Jianhong, Zhang Xuan. Construction of lentiviral vectors for solute carrier family 1 member 5 overexpression and knockdown and stably transfected RAW264.7 cell line [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1414-1421. |
| [5] | Lyu Liting, Yu Xia, Zhang Jinmei, Gao Qiaojing, Liu Renfan, Li Meng, Wang Lu. Bibliometric analysis of research process and current situation of brain aging and exosomes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1457-1465. |
| [6] | Wang Mi, Ma Shujie, Liu Yang, Qi Rui. Identification and validation of characterized gene NFE2L2 for ferroptosis in ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1466-1474. |
| [7] | Gao Yang, Qin Hewei, Liu Dandan. ACSL4 mediates ferroptosis and its potential role in atherosclerotic cardiovascular disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1239-1247. |
| [8] | Yang Bo, Pan Xinfang, Chang Liuhui, Ni Yong. Correlation of echocardiographic parameters with disability at 3 months after acute ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7544-7551. |
| [9] | Chen Ying, Guo Xiaojing, Mo Xueni, Ma Wei, Wu Shangzhi, Li Xiangling, Xie Tingting. Analysis of diagnostic biomarkers for ischemic stroke and experimental validation of targeted cuproptosis related genes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7562-7570. |
| [10] | Ping Xingfeng, Huang Zongxuan, Li Kai, Xie Guangmin, Lyu Junying. Regularity of prescriptions for ischemic stroke based on latent structure combined with association rules [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6277-6284. |
| [11] | Chen Yixin, Lu Yan, Zhang Xuan, Chen Xiaoli, Tan Liangyuan, Xu Zhangjie, Chen Wanglong, Su Shaoting, Liang Jiyao, Zhou Honghai. Mechanism by which Tongan Decoction regulates synovial macrophage polarization in rats with knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5621-5631. |
| [12] | Ning Yinkuan, Liu Linzhi, Zhou Cila, Long Yubin. Rabbit bone marrow mesenchymal stem cells transfected with recombinant lentivirus and decalcified bone matrix to construct transgenic tissue engineering materials [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(23): 4851-4858. |
| [13] | Wang Qianliang, Chen Jianpeng, Wang Yuanbin, Yan Jun. Mechanism of circ05188 targeting miR-199a-5p involved in nociceptive hypersensitivity in a rat model of lumbar disc herniation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(20): 4230-4238. |
| [14] | Chen Mingwei, Yu Wenli, Xia Suhang, Chen Bin, Chen Wenzhong, Li Fengzhen, Zhou Yu, Si Wenteng. rticular cartilage injury repaired with microRNA-140 exosomes/sodium alginate/collagen hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3326-3334. |
| [15] | E Zhengkang, Xin Hongwei, Yu Qingbo, Zhang Yunshuai. miR-192-5p targets CKIP-1 to promote osteogenic differentiation of bone marrow mesenchymal stem cells in osteoporosis patients [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2641-2647. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||