Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (7): 1800-1807.doi: 10.12307/2026.537
Previous Articles Next Articles
Regulation of AMP-activated protein kinase by platelet lysate inhibits cadmium-induced neuronal apoptosis
Liu Anting, Lu Jiangtao, Zhang Wenjie, He Ling, Tang Zongsheng, Chen Xiaoling
- Department of Blood Transfusion, The First Affiliated Hospital of Wannan Medical College, Wuhu 241001, Anhui Province, China
-
Received:2024-12-06Revised:2025-04-16Accepted:2025-05-13Online:2026-03-08Published:2025-08-20 -
Contact:Chen Xiaoling, PhD, Assistant researcher, Department of Blood Transfusion, The First Affiliated Hospital of Wannan Medical College, Wuhu 241001, Anhui Province, China Tang Zongsheng, PhD, Associate professor, Department of Blood Transfusion, The First Affiliated Hospital of Wannan Medical College, Wuhu 241001, Anhui Province, China -
About author:Liu Anting, Master candidate, Department of Blood Transfusion, The First Affiliated Hospital of Wannan Medical College, Wuhu 241001, Anhui Province, China -
Supported by:Natural Science Research Project of Anhui Educational Committee, No. 2022AH051214 (to CXL); 2022 Scientific Research Foundation for Advanced Talents of Hospital, No. YR202202 (to CXL); Project for Introduction of Advanced Talents Reward Compensation of Anhui Provincial Public Medical and Health Institutions, No. GCCRC2022003 (to CXL)
CLC Number:
Cite this article
Liu Anting, Lu Jiangtao, Zhang Wenjie, He Ling, Tang Zongsheng, Chen Xiaoling. Regulation of AMP-activated protein kinase by platelet lysate inhibits cadmium-induced neuronal apoptosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1800-1807.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
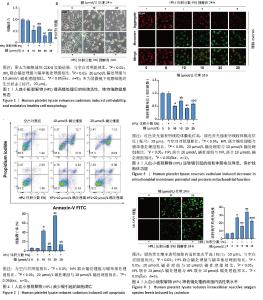
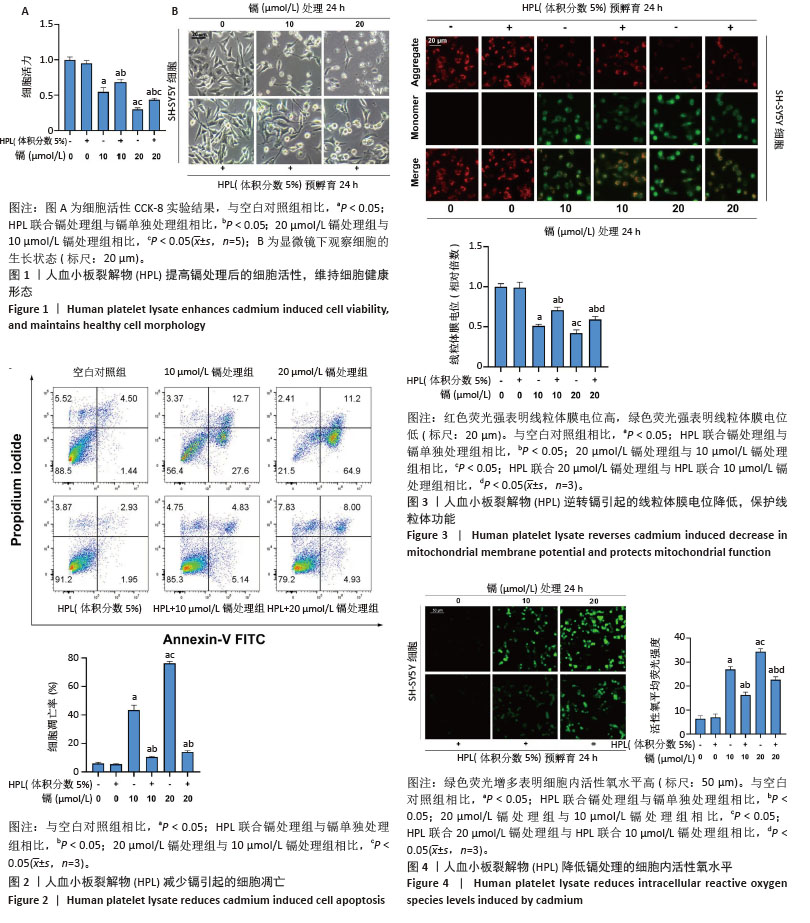
2.1 血小板裂解物对镉处理的SH-SY5Y细胞活性的影响 CCK-8检测结果显示,与空白对照组相比,镉处理后的SH-SY5Y细胞活力显著降低(P < 0.05);20 μmol/L镉处理组比10 μmol/L镉处理组的细胞活力下降更为明显(P < 0.05);而联合体积分数5%血小板裂解物处理细胞24 h可抑制镉诱导的细胞活性降低(P < 0.05),提示血小板裂解物具有显著的抗镉毒性作用,见图1A。 显微镜观察显示:空白对照组细胞呈纺锤形,细胞体较大,分支丰富且有较长的突起;与空白对照组相比,镉处理后的SH-SY5Y细胞形态显著改变,细胞皱缩,突起变短且分支减少,说明镉对神经细胞的形态结构具有显著的毒性作用;血小板裂解物组细胞形态接近空白对照组,细胞体较大,突起较长且分支丰富;与镉处理组相比,镉与血小板裂解物共同处理组细胞形态得到显著改善,细胞体增大,突起延长且分支增多,细胞整体形态更加健康,见图1B。 2.2 血小板裂解物对镉诱导的SH-SY5Y细胞凋亡的影响 使用流式细胞术检测细胞凋亡率,见图2,与空白对照组相比,镉处理组细胞凋亡水平显著升高(P < 0.05);20 μmol/L镉处理组的细胞凋亡率远高于10 μmol/L镉处理组(P < 0.05);与镉处理组相比,经血小板裂解物预处理后的SH-SY5Y细胞凋亡水平明显减少(P < 0.05)。 2.3 血小板裂解物对镉处理的细胞线粒体膜电位的影响 线粒体膜电位是反映细胞代谢和能量产生的重要指标,可以用来评估细胞的生存状态和凋亡情况。当线粒体膜电位较高时,JC-1 能够聚集在线粒体基质内,形成聚集体并发出强烈的红色荧光。相反,当线粒体膜电位下降时,JC-1无法有效聚集在基质中,主要以单体形式存在于细胞质中,此时会发出绿色荧光。与空白对照组相比,镉处理组的红/绿荧光强度比值明显降低,表示线粒体膜电位水平下降(P < 0.05);与10 μmol/L镉处理组相比,20 μmol/L镉处理组线粒体膜电位进一步降低(P < 0.05);与镉处理组相比,镉与血小板裂解物共同处理组的红/绿荧光强度比值显著提高(P < 0.05),表示线粒体膜电位水平上调,见图3。 2.4 血小板裂解物对镉诱导的SH-SY5Y细胞活性氧水平的影响 如图4所示,镉处理组细胞内的活性氧水平与空白对照组相比显著增强(P < 0.05);20 μmol/L镉处理组比10 μmol/L镉处理组表现出更高的活性氧累积(P < 0.05);经血小板裂解物干预后,细胞内的活性氧水平大幅下降(P < 0.05),表明血小板裂解物能够抑制镉诱导的SH-SY5Y细胞内活性氧含量,减轻细胞氧化应激,对细胞有保护作用。 "
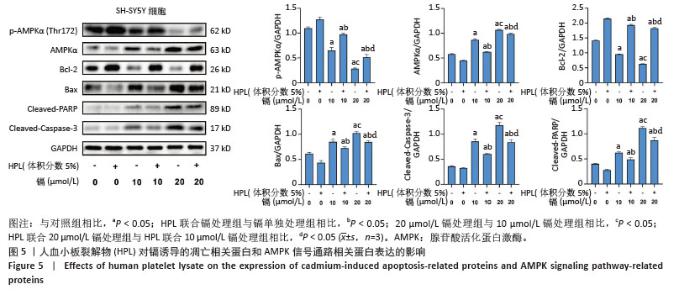
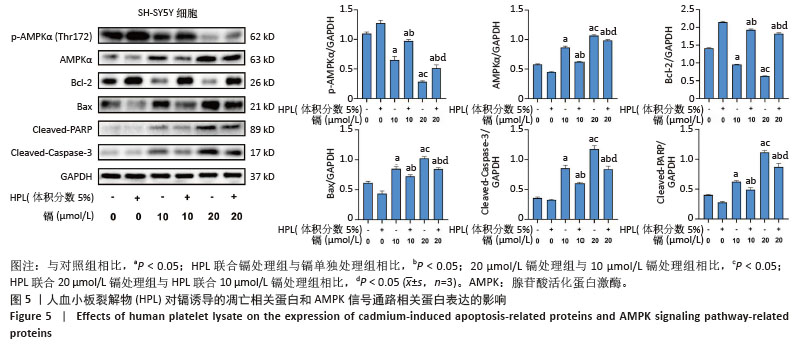
2.5 血小板裂解物对镉诱导SH-SY5Y细胞凋亡相关蛋白表达的影响 通过Western blot检测细胞中Bcl-2、Bax、Cleaved-Caspase-3和Cleaved-PARP蛋白的表达水平。如图5显示,与空白对照组相比,镉处理组细胞中凋亡蛋白Bax、Cleaved-Caspase-3和Cleaved-PARP的表达水平显著升高(P < 0.05),而抗凋亡蛋白Bcl-2的表达水平显著降低(P < 0.05);而在镉处理组的基础上,血小板裂解物预处理后显著下调了细胞中凋亡蛋白Bax、Cleaved-caspase-3和Cleaved-PARP的表达水平(P < 0.05),并上调了抗凋亡蛋白Bcl-2的表达水平(P < 0.05)。这些结果表明,血小板裂解物可能是通过调节凋亡相关蛋白的表达来抑制镉诱导的细胞凋亡。 2.6 血小板裂解物对镉诱导的SH-SY5Y细胞中AMPK信号通路的影响 通过Western blot检测细胞中p-AMPKα (Thr172)的蛋白表达水平,如图5显示,与镉处理组相比,血小板裂解物干预后上调了细胞中AMPK磷酸化水平(P < 0.05),这表明血小板裂解物能够通过促进AMPK信号通路磷酸化,提高SH-SY5Y细胞对镉诱导损伤的抵抗能力,减轻镉造成的神经细胞凋亡。 "
| [1] BARNHAM KJ, BUSH AI. Metals in Alzheimer’s and Parkinson’s diseases. Curr Opin Chem Biol. 2008;12(2):222-228. [2] REZAEI K, MASTALI G, ABBASGHOLINEJAD E, et al. Cadmium neurotoxicity: Insights into behavioral effect and neurodegenerative diseases. Chemosphere. 2024;364:143180. [3] NEWELL ME, BABBRAH A, ARAVINDAN A, et al. Prevalence rates of neurodegenerative diseases versus human exposures to heavy metals across the United States. Sci Total Environ. 2024;928:172260. [4] HAIDER FU, LIQUN C, COULTER JA, et al. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol Environ Saf. 2021;211:111887. [5] ĐUKIĆ-ĆOSIĆ D, BARALIĆ K, JAVORAC D, et al. An overview of molecular mechanisms in cadmium toxicity. Curr Opin Toxicol. 2020;19:56-62. [6] 王洋洋.镉暴露抑制自噬加剧糖尿病大鼠肾脏损伤[D].扬州:扬州大学,2023. [7] ARRUEBARRENA MA, HAWE CT, LEE YM, et al. Mechanisms of Cadmium Neurotoxicity. Int J Mol Sci. 2023;24(23):16558. [8] GUAN S, TANG M. Exposure of quantum dots in the nervous system: Central nervous system risks and the blood-brain barrier interface. J Appl Toxicol. 2024;44(7):936-952. [9] 乔梦芸,段爱茹,鱼涛,等.铅、镉、汞、砷联合暴露SH-SY5Y细胞损伤和可能机制初探[J].毒理学杂志,2024,38(4):277-285+299. [10] NEBIE O, BUÉE L, BLUM D, et al. Can the administration of platelet lysates to the brain help treat neurological disorders? Cell Mol Life Sci. 2022;79(7):379. [11] DELILA L, NEBIE O, LE NTN, et al. Neuroprotective effects of intranasal extracellular vesicles from human platelet concentrates supernatants in traumatic brain injury and Parkinson’s disease models. J Biomed Sci. 2024;31(1):87. [12] 左佳蕙,李一鸣,杨舒苇,等.血小板裂解物在再生医学和细胞治疗领域的研究进展[J].中国输血杂志,2023,36(7):642-646. [13] GOUEL F, TIMMERMAN K, GOSSET P, et al. Whole and fractionated human platelet lysate biomaterials-based biotherapy induces strong neuroprotection in experimental models of amyotrophic lateral sclerosis. Biomaterials. 2022;280:121311. [14] DELILA L, NEBIE O, LE NTN, et al. Neuroprotective activity of a virus-safe nanofiltered human platelet lysate depleted of extracellular vesicles in Parkinson’s disease and traumatic brain injury models. Bioeng Transl Med. 2022;8(1):e10360. [15] WU ZS, LUO HL, CHUANG YC, et al. Platelet Lysate Therapy Attenuates Hypoxia Induced Apoptosis in Human Uroepithelial SV-HUC-1 Cells through Regulating the Oxidative Stress and Mitochondrial-Mediated Intrinsic Apoptotic Pathway. Biomedicines. 2023;11(3):935. [16] MURALEEDHARAN R, DASGUPTA B. AMPK in the brain: its roles in glucose and neural metabolism. FEBS J. 2022;289(8):2247-2262. [17] HARDIE DG, ROSS FA, HAWLEY SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251-262. [18] XIE Z, WANG X, LUO X, et al. Activated AMPK mitigates diabetes-related cognitive dysfunction by inhibiting hippocampal ferroptosis. Biochem Pharmacol. 2023;207:115374. [19] WANG D, CAO L, ZHOU X, et al. Mitigation of honokiol on fluoride-induced mitochondrial oxidative stress, mitochondrial dysfunction, and cognitive deficits through activating AMPK/PGC-1α/Sirt3. J Hazard Mater. 2022;437:129381. [20] ZHONG S, CHEN W, WANG B, et al. Energy stress modulation of AMPK/FoxO3 signaling inhibits mitochondria-associated ferroptosis. Redox Biol. 2023;63:102760. [21] DING MR, QU YJ, HU B, et al. Signal pathways in the treatment of Alzheimer’s disease with traditional Chinese medicine. Biomed Pharmacother. 2022;152:113208. [22] CHEN L, XU B, LIU L, et al. Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radic Biol Med. 2011;50(5):624-632. [23] 霍俊凤,董爱国.不同浓度镉胁迫诱导乌龟脂质过氧化损伤的研究[J].山西中医学院学报,2019,20(1):17-19. [24] 刘振海,姚源,张振庭,等.小板裂解液对人根尖牙乳头干细胞体外增殖的影响[J].口腔颌面修复学杂志,2016,17(4):198-201. [25] SOVKOVA V, VOCETKOVA K, HEDVIČÁKOVÁ V, et al. Cellular Response to Individual Components of the Platelet Concentrate. Int J Mol Sci. 2021;22(9):4539. [26] VIAU S, LAGRANGE A, CHABRAND L, et al. A highly standardized and characterized human platelet lysate for efficient and reproducible expansion of human bone marrow mesenchymal stromal cells. Cytotherapy. 2019;21(7):738-754. [27] LEE HW, CHOI KH, KIM JY, et al. Proteomic Classification and Identification of Proteins Related to Tissue Healing of Platelet-Rich Plasma. Clin Orthop Surg. 2020;12(1):120-129. [28] OELLER M, LANER-PLAMBERGER S, KRISCH L, et al. Human Platelet Lysate for Good Manufacturing Practice-Compliant Cell Production. Int J Mol Sci. 2021;22(10):5178. [29] 谢兴琴,聂煜绮,张怡.生物材料人血小板裂解液在组织再生修复中的应用与作用[J].中国组织工程研究,2022,26(28):4553-4561. [30] PETERS K, HELMERT T, GEBHARD S, et al. Standardized Human Platelet Lysates as Adequate Substitute to Fetal Calf Serum in Endothelial Cell Culture for Tissue Engineering. Biomed Res Int. 2022;2022:3807314. [31] CHEN YC, CHUANG EY, TU YK, et al. Human platelet lysate-cultured adipose-derived stem cell sheets promote angiogenesis and accelerate wound healing via CCL5 modulation. Stem Cell Res Ther. 2024;15(1):163. [32] CENTENO C, FAUSEL Z, STEMPER I, et al. A Randomized Controlled Trial of the Treatment of Rotator Cuff Tears with Bone Marrow Concentrate and Platelet Products Compared to Exercise Therapy: A Midterm Analysis. Stem Cells Int. 2020;2020:5962354. [33] CENTENO C, SHEINKOP M, DODSON E, et al. A specific protocol of autologous bone marrow concentrate and platelet products versus exercise therapy for symptomatic knee osteoarthritis: a randomized controlled trial with 2 year follow-up. J Transl Med. 2018;16(1):355. [34] NAJAFIPOUR H, ROSTAMZADEH F, JAFARINEJAD-FARSANGI S, et al. Human platelet lysate combined with mesenchymal stem cells pretreated with platelet lysate improved cardiac function in rats with myocardial infarction. Sci Rep. 2024;14(1):27701. [35] NEBIE O, CARVALHO K, BARRO L, et al. Human platelet lysate biotherapy for traumatic brain injury: preclinical assessment. Brain. 2021;144(10):3142-3158. [36] QIU D, LI G, HU X, et al. Preclinical evaluation on human platelet lysate for the treatment of secondary injury following intracerebral hemorrhage. Brain Res Bull. 2025;220:111153. [37] GOUEL F, DO VAN B, CHOU ML, et al. The protective effect of human platelet lysate in models of neurodegenerative disease: involvement of the Akt and MEK pathways. J Tissue Eng Regen Med. 2017;11(11): 3236-3240. [38] BOORBOORI MR, ZHANG H. The effect of cadmium on soil and plants, and the influence of Serendipita indica (Piriformospora indica) in mitigating cadmium stress. Environ Geochem Health. 2024;46(11):426. [39] YAN L, CHEN C, ZHU Y, et al. Cadmium-induced phytotoxicity and tolerance response in the low-Cd accumulator of Chinese cabbage (Brassica pekinensis L.) seedlings. Int J Phytoremediation. 2021;23(13):1365-1375. [40] XU C, CHEN S, XU M, et al. Cadmium Impairs Autophagy Leading to Apoptosis by Ca2+-Dependent Activation of JNK Signaling Pathway in Neuronal Cells. Neurochem Res. 2021;46(8):2033-2045. [41] WEN S, WANG L, ZHANG C, et al. PINK1/Parkin-mediated mitophagy modulates cadmium-induced apoptosis in rat cerebral cortical neurons. Ecotoxicol Environ Saf. 2022;244:114052. [42] VELLINGIRI B, SURIYANARAYANAN A, SELVARAJ P, et al. Role of heavy metals (copper (Cu), arsenic (As), cadmium (Cd), iron (Fe) and lithium (Li)) induced neurotoxicity. Chemosphere. 2022;301:134625. [43] FEDERICO A, CARDAIOLI E, DA POZZO P, et al. Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci. 2012;322(1-2):254-262. [44] ISLAM MT. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res. 2017;39(1):73-82. [45] SAS K, ROBOTKA H, TOLDI J, et al. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci. 2007;257(1-2):221-239. [46] RENU K, CHAKRABORTY R, MYAKALA H, et al. Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium) - induced hepatotoxicity - A review. Chemosphere. 2021;271:129735. [47] AL-HASHEM FH, BASHIR SO, DAWOOD AF, et al. Vanillylacetone attenuates cadmium chloride-induced hippocampal damage and memory loss through up-regulation of nuclear factor erythroid 2-related factor 2 gene and protein expression. Neural Regen Res. 2024;19(12):2750-2759. [48] HERZIG S, SHAW RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121-135. [49] SHATI AA. Resveratrol protects against cadmium chloride-induced hippocampal neurotoxicity by inhibiting ER stress and GAAD 153 and activating sirtuin 1/AMPK/Akt. Environ Toxicol. 2019;34(12):1340-1353. |
| [1] | Yang Xuetao, Zhu Menghan, Zhang Chenxi, Sun Yimin, Ye Ling. Applications and limitations of antioxidant nanomaterials in oral cavity [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2044-2053. |
| [2] |
Dong Chunyang, Zhou Tianen, Mo Mengxue, Lyu Wenquan, Gao Ming, Zhu Ruikai, Gao Zhiwei.
Action mechanism of metformin combined with Eomecon chionantha Hance dressing in treatment of deep second-degree burn wounds#br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2001-2013.
|
| [3] | Yuan Xiaoshuang, Yang Xu, Yang Bo, Chen Xiaoxu, Tian Ting, Wang Feiqing, Li Yanju, Liu Yang, Yang Wenxiu. Effect of conditioned medium of diffuse large B-cell lymphoma cells on proliferation and apoptosis of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1632-1640. |
| [4] | He Jiale, Huang Xi, Dong Hongfei, Chen Lang, Zhong Fangyu, Li Xianhui. Acellular dermal matrix combined with adipose-derived stem cell exosomes promotes burn wound healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1699-1710. |
| [5] | Liu Huan, Zeng Shaopeng, Chen Jun, He Linqian, Yang Ying, Zhang Jing. Aging-related dysregulation of glucose metabolism: crossroads of cancer and neurodegenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1527-1538. |
| [6] | Jia Jinwen, Airefate·Ainiwaer, Zhang Juan. Effects of EP300 on autophagy and apoptosis related to allergic rhinitis in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1439-1449. |
| [7] | Liu Yu, Lei Senlin, Zhou Jintao, Liu Hui, Li Xianhui. Mechanisms by which aerobic and resistance exercises improve obesity-related cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1171-1183. |
| [8] | Wang Zhengye, Liu Wanlin, Zhao Zhenqun. Advance in the mechanisms underlying miRNAs in steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1207-1214. |
| [9] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [10] | Bao Zhuoma, Hou Ziming, Jiang Lu, Li Weiyi, Zhang Zongxing, Liu Daozhong, Yuan Lin. Effect and mechanism by which Pterocarya hupehensis skan total flavonoids regulates the proliferation, migration and apoptosis of fibroblast-like synoviocytes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 816-823. |
| [11] | Wang Zhengye, Liu Wanlin, Zhao Zhenqun. Mechanism by which vascular endothelial growth factor A targets regulation of angiogenesis in the treatment of steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 671-679. |
| [12] | Chen Ling, Mao Qiuhua, Xu Pu, Zhang Wenbo. Effect of water-soluble matrix of nano-pearl powder on proliferation, migration and apoptosis of mouse fibroblasts#br# [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 338-344. |
| [13] | Dong Chao, Zhao Mohan, Liu Yunan, Yang Zeli, Chen Leqin, Wang Lanfang. Effects of magnetic nano-drug carriers on exercise-induced muscle injury and inflammatory response in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 345-353. |
| [14] | Lu Yongheng, Zhu Shuang, Zhao Feiyan, Ai Fujun, Liu Yanjie, Dong Yangting, Guan Zhizhong, Wei Na. Effect of fluoride exposure on endoplasmic reticulum-mitochondrial calcium transfer and apoptosis in primary nerve cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 111-119. |
| [15] | Wu Zhijing, Li Jiali, Zhang Jiaxin, Wang Tangrong, Zheng Yuzhou, Sun Zixuan. Alpha-ketoglutarate engineered small extracellular vesicles delay skin aging [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 120-129. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||