Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (6): 1527-1538.doi: 10.12307/2026.603
Previous Articles Next Articles
Aging-related dysregulation of glucose metabolism: crossroads of cancer and neurodegenerative diseases
Liu Huan1, Zeng Shaopeng1, Chen Jun1, He Linqian1, Yang Ying1, Zhang Jing1, 2
- 1Department of Pathology, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China; 2National Human Brain Bank for Health and Disease, Hangzhou 310012, Zhejiang Province, China
-
Received:2025-02-06Accepted:2025-04-24Online:2026-02-28Published:2025-07-18 -
Contact:Yang Ying, Distinguished Research Fellow, Master’s supervisor, Department of Pathology, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China Co-corresponding author: Zhang Jing, Chief physician, Professor, Doctoral supervisor, Department of Pathology, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China; National Human Brain Bank for Health and Disease, Hangzhou 310012, Zhejiang Province, China -
About author:Liu Huan, MS candidate, Department of Pathology, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China -
Supported by:National Natural Science Foundation of China, Nos. 82020108012 (to ZJ) and 82371250 (to YY); Natural Science Foundation of Zhejiang Province, No. LZ23H090002 (to YY); Key Research & Development Program of Zhejiang Province, Nos. 2024C03098 (to ZJ) and 2024SSYS0018 (to ZJ [project participant])
CLC Number:
Cite this article
Liu Huan, Zeng Shaopeng, Chen Jun, He Linqian, Yang Ying, Zhang Jing. Aging-related dysregulation of glucose metabolism: crossroads of cancer and neurodegenerative diseases[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1527-1538.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
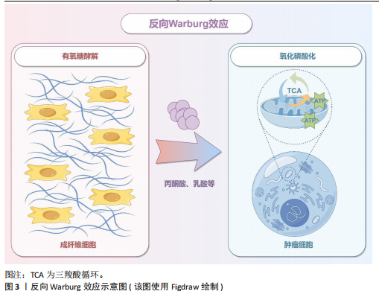
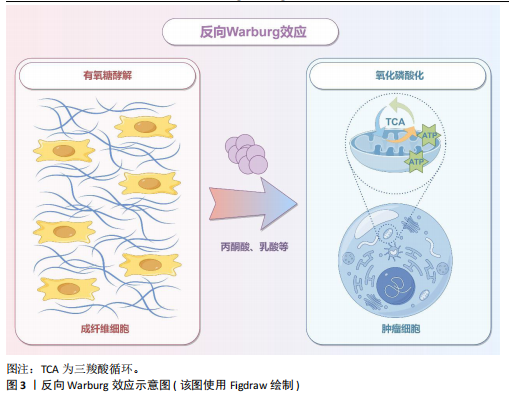
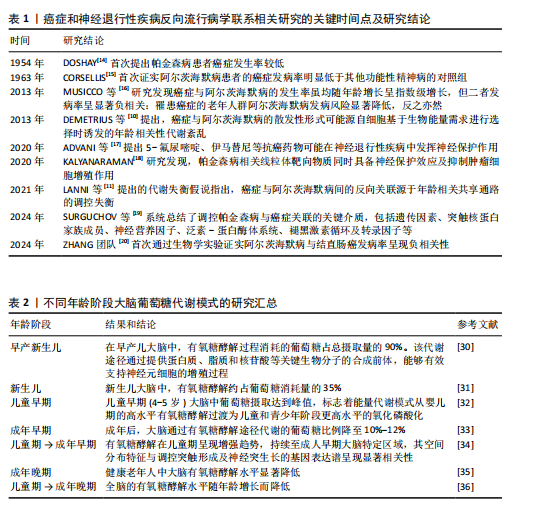
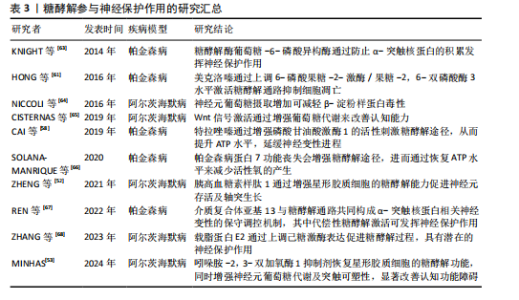
2.1 癌症和神经退行性疾病反向流行病学联系相关研究的关键时间点及研究结论 见表1。 2.2 癌症中的葡萄糖代谢重编程和乳酸化修饰 肿瘤微环境中,结缔组织异常增生和脉管系统紊乱导致氧气和营养供应不足,这种慢性代谢压力促使癌细胞通过代谢重编程实现失控增殖和侵袭转移。有氧糖酵解(即Warburg效应)是癌细胞的核心代谢特征,表现为即使在氧气充足条件下肿瘤细胞仍优先通过糖酵解分解葡萄糖并生成大量乳酸[21]。最新研究表明,癌细胞这一代谢偏好受蛋白激酶B1介导的酶空间重分布调控:蛋白激酶B1激活后通过磷酸化线粒体型苹果酸酶的Ser9位点,阻止其进入线粒体参与三羧酸循环,同时滞留在胞质的苹果酸酶可桥接多种糖酵解酶,增加糖酵解通量,进一步诱导三羧酸循环向糖酵解的代谢偏移[22]。乳酸不仅是代谢副产物,还可作为表观遗传调控的关键介质——通过单羧酸转运体1/4进入细胞核的乳酸能够介导组蛋白与非组蛋白的乳酸化修饰,从而动态关联代谢信号与表观遗传调控[23]。例如,组蛋白H3第18位赖氨酸乳酸化可上调苏氨酸酪氨酸激酶表达,激活乳酸脱氢酶A并促进乳酸生成,形成糖酵解-乳酸化正反馈循环,促进胰腺导管腺癌恶性进展[24]。另外,丙氨酰-tRNA合成酶可作为乳酸传感器感知微环境代谢变化,并催化肿瘤抑制因子p53的乳酸化修饰,从而削弱其抑癌功能[25]。上述研究提示乳酸化修饰通过整合代谢异常与表观遗传信号,已成为癌症恶性进展的关键调控枢纽。 值得注意的是,肿瘤微环境中基质细胞与肿瘤细胞可以通过反向Warburg效应形成代谢耦合[26],这一模式的核心在于细胞间的代谢分工:基质细胞(如成纤维细胞)通过有氧糖酵解产生大量乳酸及丙酮酸,而邻近的肿瘤细胞则摄取这些代谢物,通过线粒体三羧酸循环和氧化磷酸化高效合成ATP。这种类似于宿主-寄生关系的代谢互补模式(图3),不仅解释了肿瘤细胞线粒体呼吸活性升高与糖酵解活性降低的矛盾现象,还与化疗耐药密切相关。MARTINEZ-OUTSCHOORN团队[27]在乳腺癌共培养模型中发现,成纤维细胞通过反向Warburg效应诱导肿瘤细胞对他莫昔芬耐药,而联合应用他莫昔芬与达沙替尼可阻断这种代谢耦合,逆转耐药表型。近期研究进一步验证了协同干预该代谢模式的治疗优势,如WU团队[28]开发的程序化纳米系统通过同步靶向胰腺导管腺癌细胞及其主要基质细胞(活化胰腺星状细胞)的代谢异常,显著提升了治疗效果。 2.3 大脑有氧糖酵解的代谢特征和乳酸功能重塑 大脑作为人体能耗最高的器官,其能量消耗约占全身总能耗的20%,与肿瘤组织类似,这一高ATP需求的器官同样高度依赖有氧糖酵解供能[29]。研究显示,人脑通过动态调节葡萄糖代谢模式以适应不同发育阶段的生物合成需求:新生儿期,主要依赖有氧糖酵解支持神经元快速增殖[30-31];儿童期,随着突触密度激增和髓鞘形成,有氧糖酵解达到峰值,约占总葡萄糖消耗的1/3[32];成年期,该比例下降至10%-12%并持续衰减,最终在老年期基本消失[33-36]。这种代谢轨迹反映了大脑从依赖有氧糖酵解逐渐转变为以氧化磷酸化为主的模式(表2)。 尽管神经元主要通过高效的氧化磷酸化满足高能量需求,但最新研究证实糖酵解对神经元功能维持仍不可或缺[37]。JANG团队[38]在秀丽隐杆线虫模型中发现,能量应激状态下,糖酵解酶可从细胞质弥散分布转移至突触附近,以维持突触功能。进一步研"
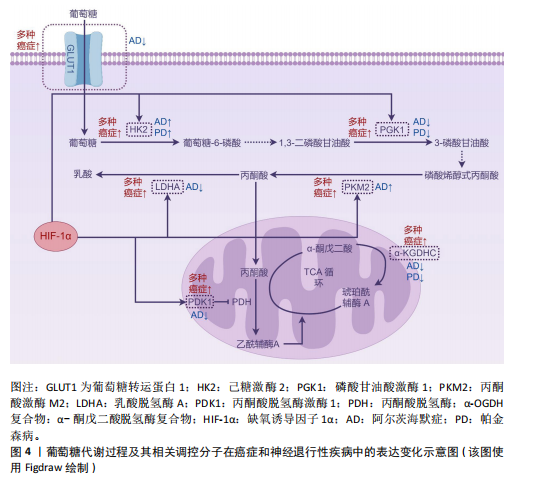
究表明,神经元的不同亚细胞结构对代谢途径存在选择性偏好:神经末梢主要依赖氧化磷酸化供能,而胞体则倾向于有氧糖酵解,这种代谢区室化既可保障能量供给,又有助于降低氧化损伤风险[39]。 星形胶质细胞的能量代谢具有显著的有氧糖酵解特征,这一特性与其胞内线粒体稀疏分布的微结构域密切相关[40]。神经元活动可瞬时激活星形胶质细胞的有氧糖酵解途径,快速生成乳酸[41]。研究发现,乳酸不仅是脑内重要的能量底物,更在长期记忆形成和认知功能维持中发挥关键调控作用,例如,DESCALZI等[42]发现当神经元表面乳酸转运载体单羧酸转运蛋白2表达下调时会导致记忆损伤,而提升内源性乳酸水平能显著增强由学习诱导的从头mRNA翻译及细胞骨架相关蛋白表达,证实乳酸对维持神经元长期记忆具有特异性代谢支持作用。另有研究进一步发现,乳酸通过增强谷氨酸/甘氨酸诱导的N-甲基-D-天冬氨酸受体依赖性内向电流及钙离子内流,激活N-甲基-D-天冬氨酸受体及其下游细胞外调节蛋白激酶信号通路,并上调神经元可塑性及活动维持相关基因c-Fos和Zif268的表达水平[43]。WANG等[44]证实脑内皮细胞单羧酸转运蛋白2表达缺陷引发的乳酸转运障碍,可导致海马神经发生障碍和认知功能衰退。另外,运动产生的乳酸通过乳酸穿梭机制进入海马区后,可通过激活沉默调节蛋白1显著提升脑源性神经营养因子表达水平,进而通过促进突触可塑性改善学习记忆功能[45]。 2.4 神经退行性疾病中的葡萄糖代谢失调 神经退行性疾病的病理进程在亚细胞层面具有高度共性,主要表现为病理性蛋白异常聚集、能量代谢紊乱及神经炎症激活等核心特征[46]。以阿尔茨海默病与帕金森病这两大常见疾病为例,虽然致病机制尚未完全阐明,但近年研究发现,通过改善葡萄糖代谢可发挥神经保护作用,这为延缓疾病进展提供了重要的干预靶点[47-48]。 阿尔茨海默病的发生发展与脑内葡萄糖代谢异常存在密切关联。影像学研究表明,患者顶叶和颞叶皮质呈现特征性葡萄糖代谢降低模式,这一特异性空间分布已成为鉴别阿尔茨海默病与其他类型痴呆的诊断标志[49]。代谢障碍不仅会直接导致突触丧失和神经元死亡,更能通过能量供应不足与神经毒性物质清除障碍形成恶性循环,加速疾病进展[50]。研究表明,神经元葡萄糖摄取下降与线粒体产能不足会削弱β-淀粉样蛋白和Tau蛋白的清除能力,而这些异常蓄积的病理蛋白又会进一步加重代谢障碍与线粒体损伤,引发能量危机并升高氧化应激水平[51]。ZHENG等[52]研究发现,胰高血糖素样肽1类似物利拉鲁肽通过激活磷脂酰肌醇3激酶/蛋白激酶B信号通路能特异性逆转β-淀粉样蛋白对星形胶质细胞糖酵解的抑制,恢复其有氧糖酵解活性,从而增强星形胶质细胞通过乳酸穿梭等途径对神经元代谢的支持能力。与之相呼应,MINHAS等[53]研究证实,β-淀粉样蛋白和Tau寡聚体通过吲哚胺-2,3-双加氧酶抑制星形胶质细胞糖酵解,而吲哚胺-2,3-双加氧酶抑制剂不仅恢复星形胶质细胞的糖酵解功能,还能通过改善神经元葡萄糖代谢和突触可塑性,最终缓解认知障碍。 帕金森病患者同样普遍存在葡萄糖代谢异常,其代谢模式具有病程依赖性特征。影像学研究显示,早期帕金森病患者主要表现为双侧苍白球、黑质及单侧尾状核和壳核葡萄糖代谢升高,同时伴随皮质代谢减退[54-55]。随着疾病进展至晚期,无论左旋多巴治疗与否,帕金森病患者顶叶、额叶、颞叶皮质及尾状核均出现葡萄糖摄取降低,这种区域性代谢改变可成为预测患者生存的重要生物学指标[56]。相关机制研究揭示,α-突触核蛋白编码基因的异常高表达会诱发糖代谢障碍,而提升葡萄糖代谢通量可有效抑制α-突触核蛋白聚集,从而阻断多巴胺能神经元凋亡通路[57-58]。基于该机制,代谢调控已成为帕金森病治疗的新方向。研究证实α1肾上腺素受体拮抗剂特拉唑嗪通过激活磷酸甘油酸激酶1促进糖酵解并增加ATP合成,显著延缓多巴胺能神经元退变进 程[59-60]。抗组胺药美克洛嗪通过增强糖酵解途径拮抗6-羟基多巴胺的神经毒性作用,为多巴胺能神经元提供保护[61];此外,胰高血糖素样肽1受体激动剂艾塞那肽通过改善糖代谢缺陷延缓疾病进展,证实胰岛素信号通路调控的治疗潜力[62]。 综合研究证据表明,葡萄糖代谢紊乱与阿尔茨海默病、帕金森病的病理进程存在显著相关性。作为葡萄糖分解的核心代谢通路,糖酵解途径的代谢通量降低不仅导致神经元能量供给不足,还会引发线粒体功能障碍并加剧氧化应激损伤。基于上述机制,靶向调节糖酵解关键酶活性以重建脑能量代谢平衡,可能为神经保护提供创新性干预策略(表3)。特别是针对糖酵解关键节点的多靶点调控方案,有望突破当前神经退行性疾病治疗领域存在的疗效瓶颈。 2.5 葡萄糖代谢参与癌症与神经退行性疾病之间的联系 2.5.1 缺氧诱导因子1的功能可塑性? 缺氧诱导因子1作为氧稳态与能量代谢的核心调控枢纽,在不同病理状态下呈现出显著的功能异质性:在肿瘤微环境中,缺氧诱导因子1通过代谢重编程等机制促进癌症进展;而在阿尔茨海默病、帕金森病等神经退行性疾病中,缺氧诱导因子1通过增强糖酵解活性和减少病理性蛋白聚集发挥神经保护作用。这种功能可塑性为阐释癌症与神经退行性疾病间的反向共病现象提供了关键理论依据[69]。 缺氧诱导因子1α作为肿瘤代谢重编程的核心调控元件,在多数恶性肿瘤中异常高表达并受多维调控网络精密调控。缺氧诱导因子1α介导的糖酵解代谢重编程不仅促进肿瘤侵袭转移,还能诱导化疗耐药:在肺腺癌中,LncRNA FAM83A-AS1通过激活缺氧诱导因子1α/糖酵解轴增强肿瘤增殖及其干性特征[70];在胶质母细胞瘤中,AMP依赖的蛋白激酶-缺氧诱导因子1α通路通过上调葡萄糖源性丝氨酸合成以促进肿瘤生长[71];在卵巢癌模型中,瞬时受体电位阳离子通道亚家族M成员7基因的沉默可通过AMP依赖的蛋白激酶介导的缺氧诱导因子1α降解途径重塑葡萄糖代谢,从而抑制恶性进展[72]。另外,活性氧/磷脂酰肌醇3激酶/蛋白激酶B与Wnt/β-catenin通路的协同激活可放大缺氧诱导因子1α介导的代谢重编程效应,该机制在结直肠癌中直接介导5-氟尿嘧啶耐药[73]。"
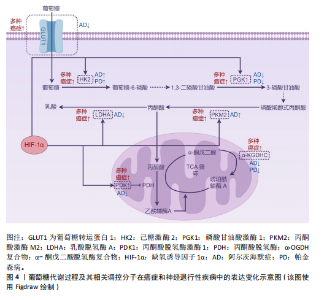
与肿瘤中的促癌效应相反,缺氧诱导因子1α在神经退行性疾病中则表现出神经保护特性。在阿尔茨海默病中,缺氧诱导因子1α通过激活糖酵解通路及血管调节相关靶基因,形成对抗氧化应激的早期适应性反应,从而延缓患者认知功能衰退[74];同时可降低β-淀粉样蛋白的神经毒性,抑制氧化应激或低氧诱导的皮质神经元凋亡[75]。研究表明,α-硫辛酸通过激活脑源性神经营养因子/酪氨酸激酶受体B信号通路上调缺氧诱导因子1α表达,进而恢复脑内葡萄糖代谢稳态[76]。对于帕金森病,缺氧诱导因子1α的靶基因具有显著神经保护作用。例如,促红细胞生成素可特异性拮抗6-羟基多巴胺对多巴胺能神经元的毒性损伤;血管内皮生长因子不仅能对抗1-甲基-4-苯基-1,2,3,6-四氢吡啶诱导的黑质细胞毒性,还可促进多巴胺能神经元增殖分化[77]。最新研究还揭示,在帕金森病模型中,药物阿苯达唑能够通过增强缺氧诱导因子1α的表达促进神经元存活,同样发挥神经保护作用[78]。 2.5.2?葡萄糖转运蛋白1的反向表达特征?葡萄糖代谢的初始限速步骤由葡萄糖转运蛋白家族介导的跨膜转运过程所决定。作为基础性亚型,葡萄糖转运蛋白1的表达水平与组织能量需求保持动态适应。值得注意的是,虽然葡萄糖转运蛋白1在癌症与阿尔茨海默病中呈现完全相反的表达模式,但最终均通过引发代谢失衡驱动疾病进展。 在肿瘤代谢重编程过程中,葡萄糖转运蛋白1作为关键葡萄糖转运体呈现显著高表达特征,其蛋白水平升高与肿瘤不良预后密切相关[79]。具体而言,肿瘤相关中性粒细胞中葡萄糖转运蛋白1的上调可促进肺癌进展并增强放疗抵抗性[80];通过抑制葡萄糖转运蛋白1可下调糖酵解活性,诱导细胞周期停滞,从而抑制肿瘤细胞增殖[81]。在RB1阳性三阴性乳腺癌中,特异性抑制葡萄糖转运蛋白1能有效阻断肿瘤生长[82]。另外,联合抑制葡萄糖转运蛋白1与氧化磷酸化可通过削弱代谢可塑性清除急性髓系白血病细胞[83]。葡萄糖转运蛋白1的表达调控涉及多通路协同作用:S100钙结合蛋白A2通过激活磷脂酰肌醇3激酶/蛋白激酶B信号通路上调葡萄糖转运蛋白1的表达,驱动糖酵解重编程进而促进肿瘤细胞增殖[84];线粒体解偶联蛋白2则通过促进葡萄糖转运蛋白1表达上调,介导胰腺癌细胞从氧化磷酸化向糖酵解的代谢表型转换[85]。相反,在阿尔茨海默病中,血脑屏障内皮细胞的葡萄糖转运蛋白1表达显著降低,这种异常可能与缺氧诱导因子1α水平下降相关[86]。研究发现,阿尔茨海默病患者脑内葡萄糖转运蛋白1水平降低不仅与Tau蛋白过度磷酸化相关,其表达减少还会加剧脑血管变性并促进β-淀粉样蛋白病理聚集,形成恶性循环的病理过程[87]。 2.5.3?磷酸甘油酸激酶1的双重生物学效应?磷酸甘油酸激酶1作为糖酵解通路的关键限速酶,催化1,3-二磷酸甘油酸转化为3-磷酸甘油酸并伴随ATP生成,其表达异常在癌症和神经退行性疾病中具有双重生物学效应。在肿瘤代谢重编程中,磷酸甘油酸激酶1对代谢表型的调控与其表达水平及亚细胞定位密切相关。在表达水平方面,磷酸甘油酸激酶1在多种恶性肿瘤中显著上调,通过促进肿瘤细胞增殖、迁移、侵袭及耐药性发挥促癌作用[88]。例如在肾透明细胞癌中,磷酸甘油酸激酶1通过激活CXC趋化因子受体4/细胞外调节蛋白激酶信号通路增强糖酵解,进而促进肿瘤发生并诱导索拉非尼耐药[89]。在亚细胞定位方面,胰腺导管腺癌研究显示,核定位的磷酸甘油酸激酶1通过线粒体氧化磷酸化主导肿瘤转移,而胞质定位的磷酸甘油酸激酶1则通过糖酵解支持细胞增殖[90];此外,线粒体易位的磷酸甘油酸激酶1可通过其蛋白激酶活性协调糖酵解与三羧酸循环[91]。与之相反,在神经退行性疾病中,抑制磷酸甘油酸激酶1的活性会加剧病理进程,而激活其活性则具有神经保护作用。在帕金森病模型中,磷酸甘油酸激酶1的表达上调可增强ATP合成,恢复突触功能并保护纹状体多巴胺轴突[92]。研究显示,药物特拉唑嗪可通过激活磷酸甘油酸激酶1增强糖酵解能力,提高ATP水平,显著延缓神经退行性变[58]。多项研究进一步证实,在多种神经退行性疾病中,激活胞质磷酸甘油酸激酶1可通过提升ATP水平溶解病理性蛋白聚集体,并通过激活自噬途径促进其降解[93]。 2.5.4?丙酮酸和乳酸代谢?丙酮酸作为糖酵解的终产物,在哺乳动物细胞中存在两种主要代谢途径:在线粒体内经丙酮酸脱氢酶复合物转化为乙酰辅酶A进入三羧酸循环,或在细胞质中被乳酸脱氢酶催化生成乳酸。其代谢方向受关键酶——丙酮酸脱氢酶激酶1和乳酸脱氢酶的精密调控。 丙酮酸脱氢酶激酶1通过磷酸化抑制丙酮酸脱氢酶复合物活性,促使代谢通向乳酸生成通路。该激酶在多种恶性肿瘤中过表达且与不良预后显著相关[94]。研究表明,丙酮酸脱氢酶激酶1表达上调可通过驱动磷脂酰肌醇3激酶/蛋白激酶B/哺乳动物雷帕霉素靶蛋白信号通路促进肝细胞癌的放疗抗性和去分化表型,而抑制丙酮酸脱氢酶激酶1则可增强鼻咽癌的放射敏感性并逆转上皮-间质转化[95-96]。 值得注意的是,在阿尔茨海默病患者大脑中观察到丙酮酸脱氢酶激酶1表达水平降低,恢复其表达不仅能减少线粒体呼吸和活性氧产生,还能增强对β-淀粉样蛋白及其他神经毒素的抵抗能力[97]。丙酮酸脱氢酶激酶1变构激动剂已被证实可改善阿尔茨海默病小鼠模型的空间学习和记忆功能[98]。 乳酸脱氢酶(乳酸脱氢酶A与乳酸脱氢酶B)的表达比例决定丙酮酸代谢走向。在肿瘤微环境中,缺氧诱导因子1α通过表观遗传重编程促使乳酸脱氢酶A优势表达,导致乳酸大量堆积[99],这种代谢重塑不仅能促进肿瘤侵袭、免疫抑制、血管生成及转移,还能通过乳酸介导的组蛋白乳酸化修饰(如H3K18la)引发肿瘤耐药[100]。具体而言,肿瘤源性乳酸通过诱导组蛋白H3K18乳酸化修饰,可促进结直肠癌中自噬增强子蛋白RUBCNL的表达,进而导致贝伐珠单抗治疗耐药[101]。靶向抑制乳酸脱氢酶A已被证实可有效逆转上述恶性表型[102]。相反,在阿尔茨海默病中,星形胶质细胞乳酸脱氢酶A表达下调可导致乳酸代谢减少,从而引发神经元能量危机[103]。 乳酸在肿瘤与神经系统中展现出双重生物学特性:在肿瘤微环境中作为促癌代谢物,通过酸化微环境、表观遗传调控等机制促进血管生成、转移及治疗抵抗[104];而在中枢神经系统则作为重要能量底物,通过调控突触长时程增强作用参与记忆巩固过 程[105]。 2.5.5?α-酮戊二酸脱氢酶复合物的代谢可塑性?α-酮戊二酸脱氢酶复合物由α-酮戊二酸脱氢酶、二氢硫辛酰胺琥珀酰转移酶、二氢硫辛酰胺脱氢酶及组装因子KGD4共同构成,通过催化α-酮戊二酸不可逆的氧化脱羧反应生成琥珀酰辅酶A。作为线粒体三羧酸循环的核心,α-酮戊二酸脱氢酶复合物处于糖、脂、氨基酸代谢的交叉调控节点,对维持细胞代谢可塑性具有关键作用。肿瘤细胞的异常增殖正依赖于这种代谢可塑性——通过重编程线粒体代谢来维持高水平的生物合成需求[106]。研究显示,在胶质母细胞瘤中α-酮戊二酸脱氢酶功能的缺失会破坏中枢碳代谢,通过激活整合应激反应和上调促凋亡蛋白Noxa,导致肿瘤细胞对Bcl-xL抑制剂的敏感性显著增加[107]。基于此机制开发的线粒体抑制剂CPI-613,可通过抑制α-酮戊二酸脱氢酶复合物活性同步阻断卵巢癌移植瘤模型中的三羧酸循环和氧化磷酸化,从而有效延长实验动物的总生存期[108]。在神经退行性疾病中,α-酮戊二酸脱氢酶复合物活性显著下降:阿尔茨海默病患者脑组织中该复合物活性降低30%-90%,且与认知功能障碍程度呈正相关;在帕金森病患者中,由二氢硫辛酰胺琥珀酰转移酶基因缺陷引发的α-酮戊二酸脱氢酶复合物活性下降,与其运动功能障碍及智力衰退密切相关[109]。研究表明,α-酮戊二酸脱氢酶复合物活性的短暂降低可能具有保护作用,但持续抑制将产生破坏性效应。而通过增强复合物活性的“有丝分裂”疗法,可能对神经退行性疾病具有潜在治疗价值[110]。 2.5.6?己糖激酶和丙酮酸激酶的矛盾现象?己糖激酶是糖酵解起始步骤的关键限速酶,负责催化葡萄糖生成葡萄糖-6-磷酸。己糖激酶2在恶性肿瘤中呈现特异性高表达,通过增强有氧糖酵解保护肿瘤细胞免于凋亡并促进免疫逃逸[111-112]。值得注意的是,阿尔茨海默病患者及模型小鼠的小胶质细胞中同样存在己糖激酶2上调现象。在病理条件下,β-淀粉样蛋白可诱导己糖激酶2过度募集至线粒体,导致小胶质细胞吞噬功能障碍及β-淀粉样蛋白的清除能力减弱[113-114];抑制己糖激酶2能增强小胶质细胞的吞噬功能并促进β-淀粉样蛋白的清除,最终改善认知障碍[114]。 阿尔茨海默病患者全脑有氧糖酵解呈现整体性下降,但不同神经细胞类型间存在显著代谢异质性:神经元和小胶质细胞的糖酵解活性异常增强,而星形胶质细胞与少突胶质细胞则表现出代谢活性减弱[115],这种差异性特征揭示了神经细胞在疾病进展中的代谢调控异质性,提示需建立多细胞互作的系统性研究框架。目前研究主要集中于小胶质细胞中己糖激酶2的表达特征,其异常升高已被证实与神经炎症等病理过程密切相关[114]。然而,神经元及星形胶质细胞等亚群中己糖激酶2的表达变化及其调控机制仍亟待阐明。通过单细胞测序等技术分析细胞特异性己糖激酶2表达谱,将为阐明代谢异质性机制提供重要突破口。综上,阿尔茨海默病脑内代谢重编程呈现整体抑制与局部激活并存的复杂特征,需从时空动态维度解析不同细胞代谢调控与病理进程的关联机制,为精准干预策略提供理论依据。 丙酮酸激酶是糖酵解过程中另一限速酶,催化磷酸烯醇式丙酮酸去磷酸化生成烯醇式丙酮酸。丙酮酸激酶M2亚型在多种恶性肿瘤中异常高表达,是维持肿瘤细胞有氧糖酵解的关键分子[116]。除代谢调控功能外,丙酮酸激酶M2亚型还参与免疫应答和DNA损伤修复等过程[117]。在阿尔茨海默病患者及模型动物中,脑内升高的丙酮酸激酶M2亚型可调节γ分泌酶活性,在缺氧微环境下促进β-淀粉样蛋白生成并诱发记忆损伤[118];此外,在糖尿病大鼠模型中,丙酮酸激酶M2亚型可介导神经元过度糖酵解,会增加糖尿病大鼠患帕金森病的风险[119]。 己糖激酶2与丙酮酸激酶M2亚型在癌症和神经退行性疾病中均呈现异常高表达并促进疾病进展,这一发现与两类疾病间存在负向关联的流行病学结论形成矛盾,提示癌症与神经退行性疾病的反向关联可能并非源于单一分子或简单机制,而涉及多分子参与的复杂调控网络。后续研究需系统解析该网络的作用机制,重点阐明这些分子在不同病理条件下的功能特异性、相互作用模式及其与相关信号通路的协同调控关系,从而为开发针对两种疾病的精准诊疗策略奠定理论基础。葡萄糖代谢过程及其相关调控分子在癌症和神经退行性疾病中的表达变化见图4。 2.6 线粒体与衰老、癌症及神经退行性疾病的交互网络 机体衰老以细胞功能进行性丧失和多组织退化为特征,导致生理功能受损并显著增加死亡风险。研究表明衰老进程与能量代谢失衡存在显著相关性,其中线粒体功能障碍会严重损害细胞能量转换效率。线粒体代谢副产物活性氧的过量蓄积通过破坏氧化磷酸化的稳态,可能引发与年龄相关的代谢紊乱,这种代谢失调最终形成恶性循环,进一步加剧线粒体功能障碍[120]。最新研究揭示,线粒体活性氧诱导的核糖体功能异常是衰老过程中代谢适应失调的重要机制[121]。在细胞命运调控中,氧化应激构成核心调控轴:生理状态下,活性氧的积累会激活核因子E2相关因子2等通路,促进超氧化物歧化酶等抗氧化酶的表达以维持氧化还原稳态[122];但当活性氧超出清除能力时,氧化应激将导致生物大分子损伤,引发细胞功能障碍并加速衰老进程[123]。 病理状态下,线粒体功能异常通过显著下调超氧化物歧化酶2等抗氧化酶,进一步增加活性氧生成[124]。肿瘤发生早期,低浓度活性氧可通过激活丝裂原活化蛋白激酶、核因子κB等促细胞增殖信号通路,同时抑制凋亡程序以增强肿瘤细胞存活。活性氧还能重塑肿瘤微环境,通过促进血管生成、炎症反应和免疫逃逸等机制驱动肿瘤进展[125]。然而,过量的活性氧具有多重危害:不仅能够引发基因组突变,还可导致蛋白质、脂质等生物分子出现不可逆氧化损伤,最终促使细胞死亡[126]。类似机制在神经退"

行性疾病中尤为显著——在阿尔茨海默病患者中,β-淀粉样蛋白沉积可诱导活性氧过度产生,导致神经元内脂质过氧化、蛋白质错误折叠及DNA损伤[127];在帕金森病中,活性氧则可特异性破坏黑质多巴胺能神经元,造成运动功能损害[128]。这些证据提示,线粒体活性氧可能构成连接癌症与神经退行性疾病的枢纽节点,靶向调控活性氧为开发跨疾病治疗策略提供了新方向。 作为线粒体质量控制的核心环节,线粒体自噬在维持细胞稳态中起重要作用,其功能失调不仅影响衰老进程,还与癌症和神经退行性疾病的发展密切相关[129-131]。研究表明,铁氧还蛋白1表达下调可通过激活线粒体自噬和磷脂酰肌醇3激酶/蛋白激酶B通路诱导活性氧生成,从而促进肝癌进展[132]。化疗和靶向药物引起的线粒体损伤可通过上调自噬水平得以缓解,这可能是导致肿瘤治疗抵抗和复发的重要机制[133]。在阿尔茨海默病中,线粒体自噬受损导致异常线粒体蓄积,促进β-淀粉样蛋白和磷酸化Tau蛋白的过度沉积,会增加神经元凋亡并阻碍能量代谢[134]。 基于上述机制,当前研究聚焦于开发靶向线粒体的跨疾病治疗策略:线粒体抗氧化剂MitoQ最初用于减少神经退行性疾病中的氧化应激损伤,最新研究显示它可抑制小鼠乳腺癌复发和肺转移[135]。激活线粒体自噬的小分子可同时延缓衰老和神经退行进程。例如,在衰老过程中,激活线粒体自噬可减少细胞内线粒体DNA依赖性的环鸟苷酸腺苷酸合酶-干扰素基因的刺激因子炎症激活,从而降低老年相关炎症和神经退行性病变[129]。但癌症中激活线粒体自噬可能促进疾病进展,如线粒体内膜锌金属蛋白酶与热休克蛋白家族A成员9竞争性结合能促进线粒体自噬并激活环鸟苷酸腺苷酸合酶-干扰素基因的刺激因子通路介导胶质母细胞瘤免疫逃逸[136]。此类干预需解决关键矛盾:增强神经元线粒体功能的同时可能激活癌细胞的代谢可塑性。因此,阐明线粒体稳态平衡机制,将成为突破现有治疗瓶颈、实现“健康衰老”和跨疾病协同治疗的关键突破口。"

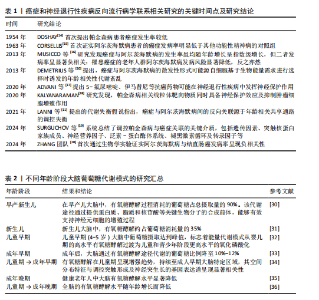
| [1] CATALA-LOPEZ F, SUAREZ-PINILLA M, SUAREZ-PINILLA P, et al. Inverse and direct cancer comorbidity in people with central nervous system disorders: a meta-analysis of cancer incidence in 577,013 participants of 50 observational studies. Psychother Psychosom. 2014;83(2):89-105. [2] ORDING AG, HORVATH-PUHO E, VERES K, et al. Cancer and risk of Alzheimer’s disease: Small association in a nationwide cohort study. Alzheimers Dement. 2020;16(7): 953-964. [3] ZHANG X, GUARIN D, MOHAMMADZADEHHONARVAR N, et al. Parkinson’s disease and cancer: a systematic review and meta-analysis of over 17 million participants. BMJ Open. 2021;11(7): e46329. [4] DONG Z, XU M, SUN X, et al. Mendelian randomization and transcriptomic analysis reveal an inverse causal relationship between Alzheimer’s disease and cancer. J Transl Med. 2023;21(1):527. [5] ZHANG Q, GUO S, ZHANG X, et al. Inverse relationship between cancer and Alzheimer’s disease: a systemic review meta-analysis. Neurol Sci. 2015;36(11): 1987-1994. [6] KIM SY, CHOI HG, KIM YH, et al. Longitudinal study of the inverse relationship between Parkinson’s disease and cancer in Korea. NPJ Parkinsons Dis. 2023;9(1):116. [7] TABARES-SEISDEDOS R, DUMONT N, BAUDOT A, et al. No paradox, no progress: inverse cancer comorbidity in people with other complex diseases. Lancet Oncol. 2011;12(6):604-608. [8] HOMMEN F, BILICAN S, VILCHEZ D. Protein clearance strategies for disease intervention. J Neural Transm (Vienna). 2022;129(2):141-172. [9] VARELA L, GARCIA-RENDUELES MER. Oncogenic Pathways in Neurodegenerative Diseases. Int J Mol Sci. 2022;23(6):3223. [10] DEMETRIUS LA, SIMON DK. The inverse association of cancer and Alzheimer’s: a bioenergetic mechanism. J R Soc Interface. 2013;10(82):20130006. [11] LANNI C, MASI M, RACCHI M, et al. Cancer and Alzheimer’s disease inverse relationship: an age-associated diverging derailment of shared pathways. Mol Psychiatry. 2021;26(1):280-295. [12] DEMETRIUS LA, SIMON DK. An inverse-Warburg effect and the origin of Alzheimer’s disease. Biogerontology. 2012;13(6):583-594. [13] HARRIS RA, TINDALE L, CUMMING RC. Age-dependent metabolic dysregulation in cancer and Alzheimer’s disease. Biogerontology. 2014;15(6):559-577. [14] DOSHAY LJ. Problem situations in the treatment of paralysis agitans. J Am Med Assoc. 1954;156(7):680-684. [15] CUMINGS JN. Mental illness and the ageing brain. J Clin Pathol. 1963;16:98-99. [16] MUSICCO M, ADORNI F, Di SANTO S, et al. Inverse occurrence of cancer and Alzheimer disease: a population-based incidence study. Neurology. 2013;81(4):322-328. [17] ADVANI D, GUPTA R, TRIPATHI R, et al. Protective role of anticancer drugs in neurodegenerative disorders: A drug repurposing approach. Neurochem Int. 2020;140:104841. [18] KALYANARAMAN B. Teaching the basics of repurposing mitochondria-targeted drugs: From Parkinson’s disease to cancer and back to Parkinson’s disease. Redox Biol. 2020;36: 101665. [19] SURGUCHOV A, SURGUCHEV AA. Association between Parkinson’s Disease and Cancer: New Findings and Possible Mediators. Int J Mol Sci. 2024;25(7):3899. [20] ZHANG N, ZHANG R, JIANG L, et al. Inhibition of colorectal cancer in Alzheimer’s disease is mediated by gut microbiota via induction of inflammatory tolerance. Proc Natl Acad Sci U S A. 2024;121(37):e1980630175. [21] WANG Y, PATTI GJ. The Warburg effect: a signature of mitochondrial overload. Trends Cell Biol. 2023;33(12):1014-1020. [22] CHEN T, XIE S, CHENG J, et al. AKT1 phosphorylation of cytoplasmic ME2 induces a metabolic switch to glycolysis for tumorigenesis. Nat Commun. 2024; 15(1):686. [23] ZHANG Y, SONG H, LI M, et al. Histone lactylation bridges metabolic reprogramming and epigenetic rewiring in driving carcinogenesis: Oncometabolite fuels oncogenic transcription. Clin Transl Med. 2024;14(3):e1614. [24] LI F, SI W, XIA L, et al. Positive feedback regulation between glycolysis and histone lactylation drives oncogenesis in pancreatic ductal adenocarcinoma. Mol Cancer. 2024; 23(1):90. [25] ZONG Z, XIE F, WANG S, et al. Alanyl-tRNA synthetase, AARS1, is a lactate sensor and lactyltransferase that lactylates p53 and contributes to tumorigenesis. Cell. 2024;187(10):2375-2392. [26] SUN K, TANG S, HOU Y, et al. Oxidized ATM-mediated glycolysis enhancement in breast cancer-associated fibroblasts contributes to tumor invasion through lactate as metabolic coupling. EBioMedicine. 2019;41:370-383. [27] MARTINEZ-OUTSCHOORN UE, LIN Z, KO Y, et al. Understanding the metabolic basis of drug resistance: therapeutic induction of the Warburg effect kills cancer cells. Cell Cycle. 2011;10(15):2521-2528. [28] WU B, WANG Z, LIU J, et al. Dual rectification of metabolism abnormality in pancreatic cancer by a programmed nanomedicine. Nat Commun. 2024;15(1):10526. [29] VANDER HEIDEN MG, CANTLEY LC, THOMPSON CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029-1033. [30] POWERS WJ, ROSENBAUM JL, DENCE CS, et al. Cerebral glucose transport and metabolism in preterm human infants. J Cereb Blood Flow Metab. 1998;18(6):632-638. [31] SETTERGREN G, LINDBLAD BS, PERSSON B. Cerebral blood flow and exchange of oxygen, glucose, ketone bodies, lactate, pyruvate and amino acids in infants. Acta Paediatr Scand. 1976;65(3):343-353. [32] BAUERNFEIND AL, BARKS SK, DUKA T, et al.Aerobic glycolysis in the primate brain: reconsidering the implications for growth and maintenance. Brain Struct Funct. 2014;219(4):1149-1167. [33] BOYLE PJ, SCOTT JC, KRENTZ AJ, et al. Diminished brain glucose metabolism is a significant determinant for falling rates of systemic glucose utilization during sleep in normal humans. J Clin Invest. 1994; 93(2):529-535. [34] GOYAL MS, HAWRYLYCZ M, MILLER JA, et al. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014;19(1): 49-57. [35] DASTUR DK. Cerebral blood flow and metabolism in normal human aging, pathological aging, and senile dementia. J Cereb Blood Flow Metab. 1985;5(1):1-9. [36] GOYAL MS, VLASSENKO AG, BLAZEY TM, et al. Loss of Brain Aerobic Glycolysis in Normal Human Aging. Cell Metab. 2017;26(2):353-360. [37] LI H, GUGLIELMETTI C, SEI YJ, et al. Neurons require glucose uptake and glycolysis in vivo. Cell Rep. 2023;42(4):112335. [38] JANG S, NELSON J C, BEND EG, et al. Glycolytic Enzymes Localize to Synapses under Energy Stress to Support Synaptic Function. Neuron. 2016;90(2):278-291. [39] WEI Y, MIAO Q, ZHANG Q, et al. Aerobic glycolysis is the predominant means of glucose metabolism in neuronal somata, which protects against oxidative damage. Nat Neurosci. 2023;26(12):2081-2089. [40] BARROS LF, BROWN A, SWANSON RA. Glia in brain energy metabolism: A perspective. Glia. 2018;66(6): 1134-1137. [41] LI X, YANG Y, ZHANG B, et al. Lactate metabolism in human health and disease. Signal Transduct Target Ther. 2022;7(1):305. [42] DESCALZI G, GAO V, STEINMAN M Q, et al.Lactate from astrocytes fuels learning-induced mRNA translation in excitatory and inhibitory neurons. Commun Biol. 2019;2:247. [43] YANG J, RUCHTI E, PETIT J, et al. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci U S A. 2014;111(33): 12228-12233. [44] WANG J, CUI Y, YU Z, et al. Brain Endothelial Cells Maintain Lactate Homeostasis and Control Adult Hippocampal Neurogenesis. Cell Stem Cell. 2019;25(6):754-767. [45] EL HAYEK L, KHALIFEH M, ZIBARA V, et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J Neurosci. 2019;39(13):2369-2382. [46] WILSON DMR, COOKSON MR, Van Den BOSCH L, et al. Hallmarks of neurodegenerative diseases. Cell. 2023; 186(4):693-714. [47] MENDEZ-FLORES OG, HERNANDEZ-KELLY LC, OLIVARES-BANUELOS TN, et al. Brain energetics and glucose transport in metabolic diseases: role in neurodegeneration. Nutr Neurosci. 2024; 27(10):1199-1210. [48] MCDONALD TS, LERSKIATIPHANICH T, WOODRUFF TM, et al. Potential mechanisms to modify impaired glucose metabolism in neurodegenerative disorders. J Cereb Blood Flow Metab. 2023;43(1):26-43. [49] SILVERMAN DH, SMALL GW, CHANG CY, et al. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. JAMA. 2001;286(17): 2120-2127. [50] CUNNANE SC, TRUSHINA E, MORLAND C, et al. Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat Rev Drug Discov. 2020;19(9):609-633. [51] CARBONELL F, ZIJDENBOS AP, BEDELL BJ. Spatially Distributed Amyloid-beta Reduces Glucose Metabolism in Mild Cognitive Impairment. J Alzheimers Dis. 2020;73(2):543-557. [52] ZHENG J, XIE Y, REN L, et al. GLP-1 improves the supportive ability of astrocytes to neurons by promoting aerobic glycolysis in Alzheimer’s disease. Mol Metab. 2021;47: 101180. [53] MINHAS PS, JONES JR, LATIF-HERNANDEZ A, et al. Restoring hippocampal glucose metabolism rescues cognition across Alzheimer’s disease pathologies. Science. 2024;385(6711):eabm6131. [54] EGGERS C, HILKER R, BURGHAUS L, et al. High resolution positron emission tomography demonstrates basal ganglia dysfunction in early Parkinson’s disease. J Neurol Sci. 2009;276(1-2):27-30. [55] BORGHAMMER P, CHAKRAVARTY M, JONSDOTTIR KY, et al. Cortical hypometabolism and hypoperfusion in Parkinson’s disease is extensive: probably even at early disease stages. Brain Struct Funct. 2010;214(4):303-317. [56] BRUMBERG J, BLAZHENETS G, BUHLER S, et al. Cerebral Glucose Metabolism Is a Valuable Predictor of Survival in Patients with Lewy Body Diseases. Ann Neurol. 2024;96(3): 539-550. [57] ZAMBON F, CHERUBINI M, FERNANDES HJR, et al. Cellular alpha-synuclein pathology is associated with bioenergetic dysfunction in Parkinson’s iPSC-derived dopamine neurons. Hum Mol Genet. 2019;28(12):2001-2013. [58] CAI R, ZHANG Y, SIMMERING JE, et al. Enhancing glycolysis attenuates Parkinson’s disease progression in models and clinical database. J Clin Invest. 2019;129(10):4539-4549. [59] RILEY MJ, MITCHELL CC, ERNST SE, et al. A model for stimulation of enzyme activity by a competitive inhibitor based on the interaction of terazosin and phosphoglycerate kinase . Proc Natl Acad Sci U S A. 2024;121(9):e1976011175. [60] SIMMERING JE, WELSH MJ, LIU L, et al. Association of Glycolysis-Enhancing alpha-1 Blockers With Risk of Developing Parkinson Disease. JAMA Neurol. 2021;78(4):407-413. [61] HONG CT, CHAU K, SCHAPIRA AHV. Meclizine-induced enhanced glycolysis is neuroprotective in Parkinson disease cell models. Sci Rep. 2016;6:25344. [62] ATHAUDA D, MACLAGAN K, SKENE SS, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017; 390(10103):1664-1675. [63] KNIGHT AL, YAN X, HAMAMICHI S, et al. The glycolytic enzyme, GPI, is a functionally conserved modifier of dopaminergic neurodegeneration in Parkinson’s models. Cell Metab. 2014;20(1):145-157. [64] NICCOLI T, CABECINHA M, TILLMANN A, et al. Increased Glucose Transport into Neurons Rescues Abeta Toxicity in Drosophila. Curr Biol. 2016;26(17):2291-2300. [65] CISTERNAS P, ZOLEZZI JM, MARTINEZ M, et al. Wnt-induced activation of glucose metabolism mediates the in vivo neuroprotective roles of Wnt signaling in Alzheimer disease. J Neurochem. 2019; 149(1):54-72. [66] SOLANA-MANRIQUE C, SANZ FJ, RIPOLLES E, et al. Enhanced activity of glycolytic enzymes in Drosophila and human cell models of Parkinson’s disease based on DJ-1 deficiency. Free Radic Biol Med. 2020; 158:137-148. [67] REN M, YANG Y, HENG KHY, et al. MED13 and glycolysis are conserved modifiers of alpha-synuclein-associated neurodegeneration. Cell Rep. 2022;41(12):111852. [68] ZHANG X, WU L, SWERDLOW RH, et al. Opposing Effects of ApoE2 and ApoE4 on Glycolytic Metabolism in Neuronal Aging Supports a Warburg Neuroprotective Cascade against Alzheimer’s Disease. Cells. 2023;12(3):410. [69] GIORDANO S, DARLEY-USMAR V, ZHANG J. Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biol. 2014;2:82-90. [70] CHEN Z, HU Z, SUI Q, et al. LncRNA FAM83A-AS1 facilitates tumor proliferation and the migration via the HIF-1alpha/ glycolysis axis in lung adenocarcinoma. Int J Biol Sci. 2022;18(2):522-535. [71] YUN HJ, LI M, GUO D, et al. AMPK-HIF-1alpha signaling enhances glucose-derived de novo serine biosynthesis to promote glioblastoma growth. J Exp Clin Cancer Res. 2023;42(1): 340. [72] CHEN Y, LIU L, XIA L, et al. TRPM7 silencing modulates glucose metabolic reprogramming to inhibit the growth of ovarian cancer by enhancing AMPK activation to promote HIF-1alpha degradation. J Exp Clin Cancer Res. 2022; 41(1):44. [73] DONG S, LIANG S, CHENG Z, et al. ROS/PI3K/Akt and Wnt/beta-catenin signalings activate HIF-1alpha-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J Exp Clin Cancer Res. 2022;41(1):15. [74] IYALOMHE O, SWIERCZEK S, ENWEREM N, et al. The Role of Hypoxia-Inducible Factor 1 in Mild Cognitive Impairment. Cell Mol Neurobiol. 2017;37(6):969-977. [75] CHAI X, KONG W, LIU L, et al. A viral vector expressing hypoxia-inducible factor 1 alpha inhibits hippocampal neuronal apoptosi. Neural Regen Res. 2014;9(11):1145-1153. [76] ZHANG Y, YAN X, XU S, et al. alpha-Lipoic Acid Maintains Brain Glucose Metabolism via BDNF/TrkB/HIF-1alpha Signaling Pathway in P301S Mice. Front Aging Neurosci. 2020; 12:262. [77] MITROSHINA EV, VEDUNOVA MV. The Role of Oxygen Homeostasis and the HIF-1 Factor in the Development of Neurodegeneration. Int J Mol Sci. 2024;25(9):4581. [78] KANDIL EA, SAYED RH, AHMED LA, et al. Hypoxia-inducible factor 1 alpha and nuclear-related receptor 1 as targets for neuroprotection by albendazole in a rat rotenone model of Parkinson’s disease. Clin Exp Pharmacol Physiol. 2019;46(12): 1141-1150. [79] BARRON CC, BILAN PJ, TSAKIRIDIS T, et al. Facilitative glucose transporters: Implications for cancer detection, prognosis and treatment. Metabolism. 2016;65(2): 124-139. [80] ANCEY P, CONTAT C, BOIVIN G, et al. GLUT1 Expression in Tumor-Associated Neutrophils Promotes Lung Cancer Growth and Resistance to Radiotherapy. Cancer Res. 2021;81(9):2345-2357. [81] LIU Y, CAO Y, ZHANG W, et al. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012; 11(8):1672-1682. [82] WU Q, BA-ALAWI W, DEBLOIS G, et al. GLUT1 inhibition blocks growth of RB1-positive triple negative breast cancer. Nat Commun. 2020;11(1):4205. [83] RODRIGUEZ-ZABALA M, RAMAKRISHNAN R, REINBACH K, et al. Combined GLUT1 and OXPHOS inhibition eliminates acute myeloid leukemia cells by restraining their metabolic plasticity. Blood Adv. 2023;7(18):5382-5395. [84] LI C, CHEN Q, ZHOU Y, et al. S100A2 promotes glycolysis and proliferation via GLUT1 regulation in colorectal cancer. FASEB J. 2020;34(10):13333-13344. [85] BRANDI J, CECCONI D, CORDANI M, et al.The antioxidant uncoupling protein 2 stimulates hnRNPA2/B1, GLUT1 and PKM2 expression and sensitizes pancreas cancer cells to glycolysis inhibition. Free Radic Biol Med. 2016;101:305-316. [86] GLUCHOWSKA K, PLISZKA M, SZABLEWSKI L. Expression of glucose transporters in human neurodegenerative diseases. Biochem Biophys Res Commun. 2021;540:8-15. [87] LIU Y, LIU F, IQBAL K, et al. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett. 2008;582(2):359-364. [88] ZHANG K, SUN L, KANG Y. Regulation of phosphoglycerate kinase 1 and its critical role in cancer. Cell Commun Signal. 2023; 21(1):240. [89] HE Y, WANG X, LU W, et al. PGK1 contributes to tumorigenesis and sorafenib resistance of renal clear cell carcinoma via activating CXCR4/ERK signaling pathway and accelerating glycolysis. Cell Death Dis. 2022;13(2):118. [90] LIANG C, SHI S, QIN Y, et al. Localisation of PGK1 determines metabolic phenotype to balance metastasis and proliferation in patients with SMAD4-negative pancreatic cancer. Gut. 2020;69(5):888-900. [91] LI X, JIANG Y, MEISENHELDER J, et al. Mitochondria-Translocated PGK1 Functions as a Protein Kinase to Coordinate Glycolysis and the TCA Cycle in Tumorigenesis. Mol Cell. 2016;61(5):705-719. [92] KOKOTOS AC, ANTONIAZZI AM, UNDA SR, et al. Phosphoglycerate kinase is a central leverage point in Parkinson’s disease-driven neuronal metabolic deficits. Sci Adv. 2024;10(34):eadn6016. [93] CHEN H, LI Y, GAO J, et al. Activation of Pgk1 Results in Reduced Protein Aggregation in Diverse Neurodegenerative Conditions. Mol Neurobiol. 2023;60(9):5090-5101. [94] ANWAR S, SHAMSI A, MOHAMMAD T, et al. Targeting pyruvate dehydrogenase kinase signaling in the development of effective cancer therapy. Biochim Biophys Acta Rev Cancer. 2021;1876(1):188568. [95] BAMODU OA, CHANG H, ONG J, et al. Elevated PDK1 Expression Drives PI3K/AKT/MTOR Signaling Promotes Radiation-Resistant and Dedifferentiated Phenotype of Hepatocellular Carcinoma. Cells. 2020; 9(3):746. [96] ZHANG W, LI L, GUO E, et al. Inhibition of PDK1 enhances radiosensitivity and reverses epithelial-mesenchymal transition in nasopharyngeal carcinoma. Head Neck. 2022;44(7): 1576-1587. [97] NEWINGTON JT, RAPPON T, ALBERS S, et al. Overexpression of pyruvate dehydrogenase kinase 1 and lactate dehydrogenase A in nerve cells confers resistance to amyloid beta and other toxins by decreasing mitochondrial respiration and reactive oxygen species production. J Biol Chem. 2012;287(44):37245-37258. [98] QUERFURTH H, SLITT A, DICAMILLO A, et al. A PDK-1 allosteric agonist improves spatial learning and memory in a betaAPP/PS-1 transgenic mouse-high fat diet intervention model of Alzheimer’s disease. Behav Brain Res. 2023;438:114183. [99] IVAN M, FISHEL ML, TUDORAN OM, et al. Hypoxia signaling: Challenges and opportunities for cancer therapy. Semin Cancer Biol. 2022;85:185-195. [100] CHEN L, HUANG L, GU Y, et al. Lactate-Lactylation Hands between Metabolic Reprogramming and Immunosuppression. Int J Mol Sci. 2022;23(19):11943. [101] LI W, ZHOU C, YU L, et al. Tumor-derived lactate promotes resistance to bevacizumab treatment by facilitating autophagy enhancer protein RUBCNL expression through histone H3 lysine 18 lactylation (H3K18la) in colorectal cancer. Autophagy. 2024;20(1):114-130. [102] SHARMA D, SINGH M, RANI R. Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics. Semin Cancer Biol. 2022;87:184-195. [103] RYU W, BORMANN M K, SHEN M, et al. Brain cells derived from Alzheimer’s disease patients have multiple specific innate abnormalities in energy metabolism. Mol Psychiatry. 2021;26(10):5702-5714. [104] QUINN WJR, JIAO J, TESLAA T, et al. Lactate Limits T Cell Proliferation via the NAD(H) Redox State. Cell Rep. 2020;33(11):108500. [105] NEWMAN LA, KOROL DL, GOLD PE. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS One. 2011;6(12):e28427. [106] VATRINET R, LEONE G, De LUISE M, et al. The alpha-ketoglutarate dehydrogenase complex in cancer metabolic plasticity. Cancer Metab. 2017;5(2):3. [107] NGUYEN TT, TORRINI C, SHANG E, et al. OGDH and Bcl-xL loss causes synthetic lethality in glioblastoma. JCI Insight. 2024; 9(8):e172565. [108] UDUMULA MP, RASHID F, SINGH H, et al. Targeting mitochondrial metabolism with CPI-613 in chemoresistant ovarian tumors. J Ovarian Res. 2024;17(1):226. [109] HANSEN GE, GIBSON GE. The alpha-Ketoglutarate Dehydrogenase Complex as a Hub of Plasticity in Neurodegeneration and Regeneration. Int J Mol Sci. 2022;23(20): 12403. [110] CHEN H, DENTON TT, XU H, et al. Reductions in the mitochondrial enzyme alpha-ketoglutarate dehydrogenase complex in neurodegenerative disease - beneficial or detrimental? J Neurochem. 2016;139(5):823-838. [111] LEE H, LI C, RUAN D, et al. Non-proteolytic ubiquitination of Hexokinase 2 by HectH9 controls tumor metabolism and cancer stem cell expansion. Nat Commun. 2019; 10(1):2625. [112] GUO D, TONG Y, JIANG X, et al. Aerobic glycolysis promotes tumor immune evasion by hexokinase2-mediated phosphorylation of IkappaBalpha. Cell Metab. 2022;34(9): 1312-1324. [113] FAIRLEY LH, LAI KO, WONG JH, et al. Mitochondrial control of microglial phagocytosis by the translocator protein and hexokinase 2 in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2023; 120(8):e2085790176. [114] LENG L, YUAN Z, PAN R, et al. Microglial hexokinase 2 deficiency increases ATP generation through lipid metabolism leading to beta-amyloid clearance. Nat Metab. 2022;4(10): 1287-1305. [115] WU Y, YANG L, JIANG W, et al. Glycolytic dysregulation in Alzheimer’s disease: unveiling new avenues for understanding pathogenesis and improving therapy. Neural Regen Res. 2025;20(8):2264-2278. [116] ZHU S, GUO Y, ZHANG X, et al. Pyruvate kinase M2 (PKM2) in cancer and cancer therapeutics. Cancer Lett. 2021;503:240-248. [117] UPADHYAY S, KHAN S, HASSAN M I. Exploring the diverse role of pyruvate kinase M2 in cancer: Navigating beyond glycolysis and the Warburg effect. Biochim Biophys Acta Rev Cancer. 2024;1879(3):189089. [118] HAN J, HYUN J, PARK J, et al. Aberrant role of pyruvate kinase M2 in the regulation of gamma-secretase and memory deficits in Alzheimer’s disease. Cell Rep. 2021;37(10): 110102. [119] LIAN B, ZHANG J, YIN X, et al. SIRT1 improves lactate homeostasis in the brain to alleviate parkinsonism via deacetylation and inhibition of PKM2. Cell Rep Med. 2024;5(8):101684. [120] TIAN Y, TIAN Y, YUAN Z, et al. Iron Metabolism in Aging and Age-Related Diseases. Int J Mol Sci. 2022;23(7):3612. [121] SNIECKUTE G, RYDER L, VIND AC, et al. ROS-induced ribosome impairment underlies ZAKalpha-mediated metabolic decline in obesity and aging. Science. 2023;382(6675): eadf3208. [122] SIES H, JONES DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol, 2020,21(7): 363-383. [123] WU Z, QU J, ZHANG W, et al. Stress, epigenetics, and aging: Unraveling the intricate crosstalk. Mol Cell. 2024;84(1):34-54. [124] KASAI S, SHIMIZU S, TATARA Y, et al. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules. 2020;10(2):320. [125] HAYES JD, DINKOVA-KOSTOVA AT, TEW KD. Oxidative Stress in Cancer. Cancer Cell. 2020;38(2):167-197. [126] CHEUNG EC, VOUSDEN KH. The role of ROS in tumour development and progression. Nat Rev Cancer. 2022;22(5):280-297. [127] AHMAD W, IJAZ B, SHABBIRI K, et al. Oxidative toxicity in diabetes and Alzheimer’s disease: mechanisms behind ROS/ RNS generation. J Biomed Sci. 2017; 24(1):76. [128] DIONISIO PA, AMARAL JD, RODRIGUES CMP. Oxidative stress and regulated cell death in Parkinson’s disease. Ageing Res Rev. 2021;67:101263. [129] JIMENEZ-LOYGORRI JI, VILLAREJO-ZORI B, VIEDMA-POYATOS A, et al. Mitophagy curtails cytosolic mtDNA-dependent activation of cGAS/STING inflammation during aging. Nat Commun. 2024;15(1):830. [130] AMARAVADI RK, KIMMELMAN AC, DEBNATH J. Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 2019;9(9):1167-1181. [131] YANG K, YAN Y, YU A, et al. Mitophagy in neurodegenerative disease pathogenesis. Neural Regen Res. 2024;19(5):998-1005. [132] SUN B, DING P, SONG Y, et al. FDX1 downregulation activates mitophagy and the PI3K/AKT signaling pathway to promote hepatocellular carcinoma progression by inducing ROS production. Redox Biol. 2024;75:103302. [133] FAN S, PRICE T, HUANG W, et al. PINK1-Dependent Mitophagy Regulates the Migration and Homing of Multiple Myeloma Cells via the MOB1B-Mediated Hippo-YAP/TAZ Pathway. Adv Sci (Weinh). 2020;7(5):1900860. [134] BELL SM, BARNES K, DE MARCO M, et al. Mitochondrial Dysfunction in Alzheimer’s Disease: A Biomarker of the Future? Biomedicines. 2021;9(1):63. [135] CAPELOA T, KRZYSTYNIAK J, RODRIGUEZ AC, et al. MitoQ Prevents Human Breast Cancer Recurrence and Lung Metastasis in Mice. Cancers (Basel). 2022;14(6):1488. [136] ZHU WD, RAO J, ZHANG LH, et al. OMA1 competitively binds to HSPA9 to promote mitophagy and activate the cGAS-STING pathway to mediate GBM immune escape. J Immunother Cancer. 2024;12(4):e8718. |
| [1] | Leng Xiaoxuan, Zhao Yuxin, Liu Xihua. Effects of different neuromodulatory stimulation modalities on non-motor symptoms in Parkinson’s patients: a network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1282-1293. |
| [2] | He Qiwang, , , Chen Bo, Liang Fuchao, Kang Zewei, Zhou Yuan, Ji Anxu, Tang Xialin, . Relationship between Alzheimer’s disease and sarcopenia and body mass index: analysis of GWAS datasets for European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1036-1046. |
| [3] | Xie Liugang, Cui Shuke, Guo Nannan, Li Aoyu, Zhang Jingrui. Research hotspots and frontiers of stem cells for Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1475-1485. |
| [4] |
Li Tian, Ren Yuhua, Gao Yanping, Su Qiang.
Mechanism of agomelatine alleviating anxiety- and depression-like behaviors in APP/PS1 transgenic mice #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1176-1182.
|
| [5] | Lu Ranran, Zhou Xu, Zhang Lijie, Yang Xinling. Dimethyl fumarate alleviates nerve damage in a mouse model of Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 989-994. |
| [6] | Su Qin, Jia Siwei, Guo Minfang, Meng Tao, Li Yanbing, Mu Bingtao, Song Lijuan, Ma Cungen, Yu Jiezhong. Lycium barbarum polysaccharide intervenes in SH-SY5Y cell injury induced by beta-amyloid protein 1-42: protective effect of mitochondrial autophagy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6688-6696. |
| [7] | Cui Yuena, Chen Xiaoyu, Liang Meiting, Chen Wujin, He Yi, Dilinur·Ekpa, Du Manxi, Zhu Yuqiu, Abuduwupuer·Haibier, Sun Yuping. Differences of calorie restriction and time-restricted feeding on metabolic indices and gut microbiota of mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6449-6456. |
| [8] | Zhang Xin, Guo Baojuan, Xu Huixin, Shen Yuzhen, Yang Xiaofan, Yang Xufang, Chen Pei. Protective effects and mechanisms of 3-N-butylphthalide in Parkinson’s disease cell models [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6466-6473. |
| [9] | Liu Ruojing, Zhao Xue, Zhu Yizhen, Fu Lingling, Zhu Junde. Ginsenoside Rb1 alleviates cerebral ischemic injury in mice by regulating microglial polarization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6219-6227. |
| [10] | Nan Songhua, Peng Chaojie, Cui Yinglin. Mitochondrial dysfunction and brain aging: a bibliometrics analysis based on the Web of Science Core Collection database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5642-5651. |
| [11] | Jiang Qianping, Yang Dan, , , Wan Shilei, Xu Dandan, , , , Cao Lu, , Zhou Jing, , , . Role of O-linked N-acetylglucosamine glycosylation in neurodegenerative diseases and its clinical application prospects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5704-5712. |
| [12] | He Ningjuan, Li Li, Wang Su, Yang Jianshe, Lei Siyun, Wang Yang. Effects of aerobic or resistance exercise on hippocampal ras/Drebrin dendritic spine plasticity in a mouse model of Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5528-5535. |
| [13] | Shen Zilong, Wu Mingjie, Chen Xiaojing, Zhou Xibin, Zhou Chunxiang. An experimental method for simultaneously extracting the dura mater and deep cervical lymph nodes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5543-5548. |
| [14] | Zhu Liuhui, Zhang Xinyue, Zhu Zhouhai, Yang Xinglong, Guan Ying, Liu Bin. Coiled-coil-helix-coiled-coil-helix domain-containing 2 inhibits apoptosis of Parkinson’ s disease SH-SY5Y cells by promoting mitochondrial autophagy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5403-5413. |
| [15] | Lei Senlin, Chen Xiaoan, Chen Ping, Wang Zhaofeng. Exercise prevention and treatment of Parkinson’ s disease mediated by brain-derived neurotrophic factor: role and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5454-5468. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||