Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (9): 2334-2342.doi: 10.12307/2026.645
Previous Articles Next Articles
Lumbar fusion combined with unilateral fixation for lumbar degenerative diseases: biomechanics, technical evolution, and clinical applications
Zhang Xianxu, Ma Zhong, Liu Xin, Huang Lei, Shen Wenxiang, Luo Zhiqiang
- Department of Orthopedics, The Second Hospital of Lanzhou University, Lanzhou 730030, Gansu Province, China
-
Received:2025-03-03Accepted:2025-05-15Online:2026-03-28Published:2025-09-29 -
Contact:Luo Zhiqiang, PhD, Chief physician, Department of Orthopedics, The Second Hospital of Lanzhou University, Lanzhou 730030, Gansu Province, China -
About author:Zhang Xianxu, Physician, Department of Orthopedics, The Second Hospital of Lanzhou University, Lanzhou 730030, Gansu Province, China -
Supported by:Cuiying Science and Technology Innovation Project of Second Hospital of Lanzhou University, No. CY2021-MS-A03 (to LZQ)
CLC Number:
Cite this article
Zhang Xianxu, Ma Zhong, Liu Xin, Huang Lei, Shen Wenxiang, Luo Zhiqiang . Lumbar fusion combined with unilateral fixation for lumbar degenerative diseases: biomechanics, technical evolution, and clinical applications [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2334-2342.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
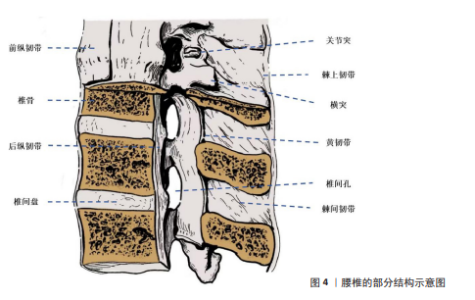
2.1 腰椎退行性疾病介绍 2.1.1 腰椎的结构和功能 腰椎是脊柱下部的重要组成部分,位于胸椎与骶椎之间,由5块椎体(L1-L5)及其相关的椎间盘、韧带、关节,以及周围的肌肉和筋膜共同构成[25-26](图4)。腰椎具有独特的解剖学特点,呈肾形的椎体较大且宽厚[27],这一解剖特征为腰椎承载上半身质量提供了良好的结构支持,并在日常活动中发挥支撑和稳定的作用。而位于椎体之间的椎间盘,其解剖结构由髓核、纤维环和软骨终板组成,具有缓冲负重、减震和分散应力的功能[28]。 腰椎主要具有前屈、后伸、侧屈及旋转等运动功能[29-31],尽管它的运动幅度小于颈椎,但腰椎的承重能力与稳定性更为突出[32-33]。腰椎的承重能力与稳定性依赖于多种结构的协调作用,主要包括椎间盘的弹性功能、椎体关节间的机械锁定、强大的韧带系统(如前纵韧带、后纵韧带、黄韧带、棘上韧带、棘间韧带等)以及附着于腰椎的多种肌群(如竖脊肌、多裂肌等)[26,29],这些结构相互协作,共同维持了腰椎的机械强度,同时赋予其一定的灵活性。 腰椎区域的神经结构复杂,主要包含马尾神经在内的多条神经根,这些神经根对下肢的感觉与运动功能以及膀胱和直肠的自主功能调节具有重要作用[26,34]。但长期的机械负荷、创伤以及老化所引起的退行性改变等因素易导致腰椎结构异常,并进一步加速腰椎退行性疾病的发生与发展[35-36]。 2.1.2 腰椎退行性疾病的病因病理和治疗现状 腰椎退行性疾病多发生于中老年人群,是以腰椎结构退行性改变为主要病理特征的脊柱疾病。目前腰椎退行性疾病的具体病因及其发病机制仍未完全阐明,学界普遍认为腰椎退行性疾病的发生是多种因素如遗传、年龄相关的椎间盘退变、长期的机械负荷以及创伤等综合作用的结果,其中遗传因素被认为是最重要的危险因素[37-38]。在病理层面,腰椎退行性疾病的主要特征是椎间盘退变、椎间隙狭窄、黄韧带肥厚以及关节突增生,这些病理改变共同导致腰椎稳定性下降,并可能诱发神经根受压及脊柱不稳。在临床表现方面,腰椎退行性疾病患者的主要症状通常以慢性腰背痛为主,并常伴随神经根性症状例如坐骨神经痛、下肢麻木或间歇性跛行等。症状严重的患者可能出现下肢无力或步态异常等表现,这些症状会显著降低患者的生活质量。 腰椎退行性疾病的治疗策略主要包括保守治疗和手术治疗[39-40]。对于症状轻微的患者常采用药物治疗、物理疗法以及生活方式干预等措施,而对于症状较为严重或长期保守治疗无效的患者则常需要进行手术干预。腰椎融合术已成为腰椎退行性疾病手术治疗的主要方式,在缓解神经压迫、恢复椎间隙高度以及重建脊柱稳定性方面表现出显著的治疗效果[14-15,41]。近年来微创技术的应用显著降低了手术创伤,并为患者提供了更加优化的治疗选择。"
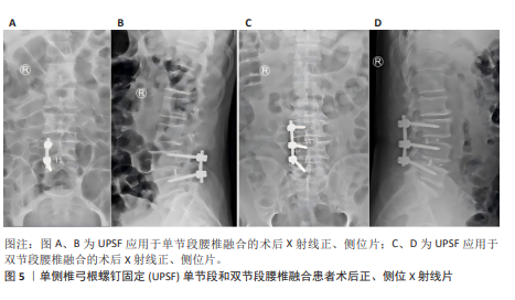
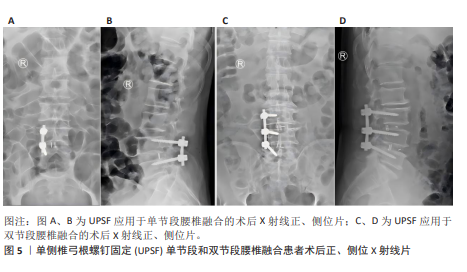
2.2 腰椎融合术联合单侧椎弓根螺钉固定的发展历程 2.2.1 腰椎融合术临床应用历程 腰椎融合术由HIBBS[42]于1911年首次提出,现在已逐渐发展为治疗腰椎退行性疾病的金标准。1952年,CLOWARD[43]提出了经后路腰椎融合术(posterior lumbar interbody fusion,PLIF),该术式在改善神经松解效果和恢复脊柱稳定性方面表现良好,但由于手术创伤较大,其应用增加了术后腰椎肌肉萎缩及并发症的风险。为改善经后路腰椎融合术的不足,HARMS等[44]于1991年提出了经椎间孔腰椎融合术(transforaminal lumbar interbody fusion,TLIF)。与经后路腰椎融合术不同,经椎间孔腰椎融合术通过椎间孔入路,显著降低了神经及硬膜囊损伤的风险,同时保留了更多脊柱后方骨性结构,从而减少了术后并发症的发生率。研究表明经椎间孔腰椎融合术在临床疗效方面与经后路腰椎融合术无显著差异,但在手术时间、术中出血量及术后恢复速度等方面优于经后路腰椎融合术[45-46]。然而传统经椎间孔腰椎融合术由于术中对椎旁肌的过度牵拉,容易导致术后腰痛以及椎旁肌功能障碍。WILTSE等提出了椎旁肌间隙单侧入路经椎间孔腰椎融合术,该术式通过肌间隙进入,避免了肌肉剥离与损伤,从而显著降低了术后腰痛的发生率[47]。 2002年,FOLEY等[48]在引入微创技术后,提出了微创经椎间孔腰椎融合术,该技术通过管状扩张器减小对脊柱后方结构的损伤,进一步减少术中失血和术后疼痛,并显著缩短住院时间。近年来,随着脊柱内镜技术的不断发展,经皮内镜下经椎间孔腰椎融合术及全内镜下腰椎融合术相继被提出,并进一步减少了术中创伤[49-50]。 研究发现经皮内镜下经椎间孔腰椎融合术与微创经椎间孔腰椎融合术在中长期疗效方面无显著差异,但在手术时间及术后恢复时间上表现出显著的缩短[51-52]。全内镜下腰椎融合术在此基础上实现了全程内镜化操作,为未来微创技术的发展提供了更多潜在可能性。在过去30余年的发展中,腰椎融合术已衍生出多种改良术式。但这些术式大多依赖于内固定手段的辅助,且当前高质量的前瞻性随机研究仍然有限,故其长期临床疗效尚需进一步验证。 2.2.2 UPSF的引入与应用 随着腰椎融合术在腰椎退行性疾病治疗中的广泛应用,BPSF长期以来被认为是标准的内固定术式。但BPSF存在多种局限性,包括手术创伤较大、手术时间较长、术中出血量较多,尤其是术后邻近节段退变发生风险较高[53-54],这些局限性促使临床实践逐渐探索更为微创的固定技术。UPSF作为一种新兴的固定方式,最早由KABINS等[55]在1992年首次报道。该术式旨在减少传统BPSF引起的手术创伤及相关并发症,同时尽可能获得与BPSF相似的生物力学稳定性和临床疗效[17,56]。UPSF能够实现与BPSF接近的融合效果,同时通过降低内固定强度,有助于避免融合阶段因过强固定导致的邻近节段退变。UPSF在腰椎融合术中的应用进一步增强了腰椎融合手术的微创性,腰椎融合术联合UPSF在手术时间及术中出血量方面表现出显著的优势。YANG等[57]的研究显示UPSF组患者的平均手术时间及术中失血量分别减少了20%-30%,并且UPSF能够保留对侧正常的脊柱结构,尤其是减少了对椎旁肌肉及韧带的损伤。研究表明其术后疼痛评分(目测类比评分)显著低于BPSF,且患者术后活动能力恢复更为迅速[58]。UPSF还能通过适度降低内固定强度,有效减少融合节段相邻椎体的生物力学应力,从而显著降低术后邻近节段退变的发生率,KIM等[59]的生物力学研究进一步证实了这一结论。所以腰椎融合术作为治疗腰椎退行性疾病的常用手术方式,通过联合UPSF,不仅能够有效缓解神经压迫和重建脊柱稳定性,还可以显著减少手术时间、术中出血量及术后并发症的发生率。 2.2.3 UPSF的创新性与多学科融合前景 近年来,UPSF作为一种新兴的固定方式,凭借其独特的生物力学性能和微创优势,已逐渐成为治疗腰椎退行性疾病的重要技术之一。UPSF的核心创新是通过单侧固定实现了微创化与生物力学稳定性的平衡。相较于传统的BPSF,UPSF显著减少了手术创伤和缩短了手术时间,并最大程度地保留了正常脊柱结构,尤其是椎旁肌和韧带,从而降低了术后软组织损伤和邻近节段退变的发生风险。从固定方式来看,UPSF使用非对称的单侧固定,一边有效分散负荷、避免应力集中,一边减少了对对侧结构的干扰,提升了术后的功能恢复与舒适度。而轻质且具良好生物相容性的钛合金螺钉是UPSF术中常用的材料,它兼具足够的力学支持和较低的术后排异风险,进一步提高了患者术后恢复质量。 随着技术的不断发展, UPSF手术与多学科新兴技术的融合也逐渐被人们所关注,并展现出广阔的临床应用前景。机器人导航技术的引入使UPSF在术前规划和术中操作方面更加精准,该技术通过三维建模与实时导航系统,可显著降低螺钉置入误差,尤其在解剖结构复杂或存在畸形的患者中表现出更高的安全性和有效性。而全内镜技术的应用进一步增强了UPSF手术的微创优势,通过更小的手术通道和可视化操作,全内镜UPSF手术在减少软组织牵拉、降低术后疼痛以及缩短恢复时间方面具有明显优势,特别适用于翻修手术或存在局部解剖粘连的复杂病例。并且3D打印融合器和人工智能术前规划系统的结合也为UPSF提供了个性化治疗的新方向,3D打印融合器可根据患者解剖结构定制尺寸和形状,优化椎间稳定性与融合效果;人工智能则可整合影像学数据与临床信息,自动生成优化的手术路径与固定方案,提升术中决策的科学性与效率。所以UPSF在微创理念、生物力学性能以及材料选择等方面具有显著创新。UPSF与机器人导航、全内镜技术、3D打印、人工智能等前沿技术的融合,正逐步推动其向更高精准度、更强个体化和更广适应证的方向发展,并展现出极大的临床推广潜力和科研价值。 2.2.4 UPSF手术技术的学习曲线与培训策略 UPSF手术在成功和推广过程中起重要作用的是术者的经验和设备条件。相较于传统的BPSF手术,UPSF技术具有较高的微创要求,术者的熟练程度和手术技巧直接影响手术的效果。手术时间和并发症发生率通常随着病例积累而逐步下降,研究表明随着手术经验的积累,术者的操作熟练度逐渐提高和手术时间逐步稳定后,术中出血量也会逐渐下降。邹海波等[60]报道单侧固定组手术时间基本上与病例数保持较好的拟合关系,而术中出血量具有显著变化,前14例患者的术中出血量为(227.14±87.74) mL,后13例患者为(134.61±82.01) mL,差异有显著性意义(t=2.825,P=0.009),所以手术时间和患者恢复的变化趋势与手术经验密切相关。为了提高UPSF的推广效率,建议对术者进行规范化培训并引入标准化的操作流程,确保术者能够在较短时间内掌握相关技术并减少操作误差。标准化操作流程应包括术前准备、手术入路、螺钉固定及术后管理等环节,确保操作的规范性和一致性。并建议开展相关培训课程及模拟手术实践,帮助术者在安全、有效的环境下掌握技术,进一步促进UPSF的临床推广。 2.3 生物力学 任何固定方式在腰椎融合术中的广泛临床应用,均需首先通过生物力学研究验证其可行性与安全性。传统研究表明腰椎融合术联合BPSF能够为融合节段提供较高的生物力学稳定性[61-62]。尽管BPSF在稳定性方面表现优越,其较大的手术创伤和邻近节段退变的风险使得UPSF成为一种有吸引力的替代方案。SAINI等[61]通过有限元模型对TLIF和PLIF联合UPSF或BPSF的生物力学特性进行了研究,结果显示BPSF在屈曲、伸展、侧屈及轴向旋转等运动模式中表现出优于UPSF的稳定性,但这种稳定性是以更大的手术创伤为代价的,且过度坚固的内固定可能促进邻近节段退变的发生。 大量研究表明,UPSF在单节段和双节段腰椎融合术中能提供足够的生物力学稳定性,并明显减小手术创伤[20,59,63-64](图5)。LI等[63]通过小牛腰椎模型对UPSF与BPSF的生物力学性能进行了系统对比研究,结果显示UPSF在轴向旋转与屈曲稳定性方面略低于BPSF,但在减少融合节段应变不对称性及降低应力集中方面表现优于传统双侧固定。而KIM等[59]的研究表明在单节段腰椎融合术中,在屈曲和伸展力矩下,与BPSF组相比UPSF组导致融合后相邻节段运动范围增加减少约50%;在拉伸和扭转力矩下,UPSF组的运动范围仍分别为47.8%和77.7%,比BPSF组16.0%和50.5%的运动范围更高;并且UPSF组模型明显降低了在屈曲、伸展、扭转和侧弯力矩下相邻L2-3节段增加的对纤维环的最大von Mises应力。这些结果都表明UPSF能够明显降低邻近节段的生物力学应力,对于减少邻近节段退变的风险具有重要意义[59]。进一步研究表明UPSF的固定稳定性与融合器的设计及配置密切相关。CHEN等[20]基于猪腰椎模型验证了UPSF结合双融合器的生物力学性能,结果表明这种组合方式在屈曲、侧屈及轴向旋转中可获得接近BPSF的稳定性。DU等[64]的研究显示通过优化融合器的高度与位置,UPSF在单节段腰椎融合中的生物力学表现甚至可达到BPSF的水平。而AMBATI等[16]基于有限元模型研究了UPSF与BPSF结合不同形状融合器的生物力学性能,进一步验证了UPSF在适当条件下的稳定性。 但UPSF在多节段融合手术中的应用仍然存在一定争议。YüCESOY等[62]基于成人尸体标本研究了UPSF在双节段融合中的生物力学特性,结果表明UPSF在双节段融合中提供的稳定性显著低于BPSF,尤其体现在轴向旋转与侧屈的应力分布方面。这一研究结果的矛盾性可能源自不同生物力学模型间的差异,尸体标本模型与有限元模拟模型之间的差异可能导致应力分布和力学行为的不同表现,而手术技术变量如融合器类型、螺钉角度等也可能在UPSF与BPSF的生物力学稳定性比较中起到重要作用。对于多节段融合手术而言,固定节段数量的增加可能要求更高的内固定强度,而这也可能是导致UPSF在多节段融合中生物力学稳定性较低的原因之一。所以UPSF在多节段复杂病变中的生物力学稳定性仍需进一步研究与验证,进一步的生物力学研究应考虑的因素包括尸体标本与有限元模拟模型的差异、固定节段数量、螺钉角度调整及融合器设计优化等,以便于提供更为全面的解释和解决方案。结合这些因素,有望为UPSF在多节段融合手术中的应用提供更为合理的生物力学解释并减少争议,从而促进其临床应用的进一步发展。 UPSF在单节段与双节段腰椎融合术中的生物力学稳定性已得到广泛验证,其在降低术后并发症及减少邻近节段退变风险方面的优势尤为突出,但UPSF在复杂融合手术中的适用性仍需进一步的生物力学研究与高质量临床试验予以证实。未来研究应进一步优化UPSF的固定结构设计,并结合患者个体化的病理特征,促进其在复杂融合手术中的广泛应用。"
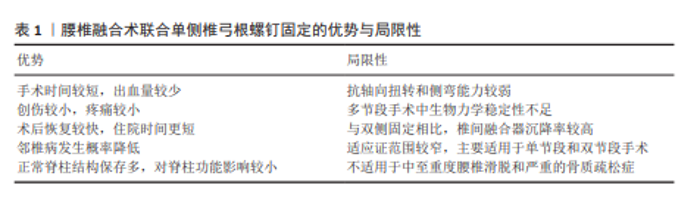
2.4 临床应用 2.4.1 临床疗效 腰椎退行性疾病严重影响中老年患者的生活质量,保守治疗无效时常需要通过手术干预。腰椎融合术联合UPSF作为一种微创手术方式,其围术期表现及术后长期疗效已在多项研究中得到验证。CHENG等[54]在一项前瞻性随机研究中,纳入了48例腰椎椎管狭窄症患者,比较了腰椎融合术联合UPSF与BPSF在术后2年的临床及影像学结果,研究结果显示两组患者的疼痛目测类比评分及Oswestry功能障碍指数均显著改善,且椎间隙高度、椎间孔高度及节段性前凸角度的恢复效果相似(均P > 0.05),但BPSF组的手术时间及术中失血量均显著高于UPSF组(均P < 0.05)。而YANG等[57]在对66例单节段腰椎退行性疾病患者的研究中发现UPSF组与BPSF组在术后目测类比评分及Oswestry功能障碍指数的改善方面无显著性差异(均P > 0.05),但UPSF组的手术时间与术中失血量分别减少了约24%和18%(均P < 0.05)。XUE等[58]开展的一项平均随访25.3个月的前瞻性随机研究表明,UPSF组在围术期表现方面优于BPSF组,而两组在总融合率、螺钉故障率及并发症发生率方面无显著性差异(均P > 0.05)。并且SUK等[65]在一项针对87例腰椎退行性疾病患者的研究中进一步验证了单侧固定的临床可行性,研究结果显示UPSF组在手术时间、住院时间及医疗费用方面均优于BPSF组(均P < 0.05),而两组在融合率、临床满意度及并发症发生率方面差异无显著性意义(均P > 0.05)。其他多项研究也表明UPSF在缓解症状、促进椎间融合及降低术后并发症方面的效果与BPSF相当,同时显著减少了手术创伤及术后恢复时间[18,66-70]。 近些年的临床研究大多只进行了术后短期效果随访(如2年随访),但对于术后长期疗效的研究仍相对缺乏,尤其是腰椎融合术联合UPSF术后的长期并发症包括螺钉松动、融合器沉降等尚未得到充分的关注。虽已有研究表明短期内UPSF组与BPSF组在疼痛缓解、功能改善等方面相似,但由于缺乏长期随访数据,关于这些手术的耐久性和长期并发症的分析仍不充分。所以未来的研究需要填补这一空白,重点关注长期并发症及手术耐久性,尤其是在骨质疏松、术后邻近节段退变等高风险患者群体中的表现。并且尽管上述研究肯定了腰椎融合术联合UPSF在腰椎退行性疾病治疗中的临床价值,但其在复杂病变中的安全性及疗效仍需通过更多高质量的随机对照研究加以验证。 2.4.2 优势与局限性 腰椎融合术联合UPSF作为一种新兴的内固定技术,在腰椎退行性疾病的治疗中展现出显著的微创优势(表1)。UPSF与传统的BPSF相比,明显缩短了手术时间和减少了术中出血量和透视次数,进而降低了围术期的风险。而且UPSF具有创伤较小的特点,可有效减少术后疼痛和软组织损伤,以便于加快患者的术后康复过程和缩短住院时间,并降低综合医疗费用。这些特点不仅使UPSF符合现代微创手术理念,同时为部分高龄患者或伴有基础疾病的患者提供了更为安全的治疗选择。UPSF还能通过降低内固定强度有助于减轻融合节段对邻近节段的应力集中,这有利于降低邻近节段退变的发生率。并且UPSF通过保留对侧正常的脊柱解剖结构,在维持脊柱稳定性与运动功能方面展现出独特优势,并且其生物力学性能能够满足单节段及部分双节段融合的临床应用需求。 但UPSF的局限性同样需要引起重视(表1)。UPSF在抗轴向扭转和侧屈应力方面的性能略低于BPSF,这种非对称固定的特性导致其在多节段融合或复杂病变患者中表现出一定的生物力学劣势。研究发现腰椎融合术联合UPSF的术后融合器沉降率高于BPSF,这可能对术后脊柱稳定性产生潜在的不利影响,尤其是在骨质疏松或椎间盘高度显著丧失的患者中表现更为显著。UPSF的适应证相对较为局限,主要适用于单节段或特定双节段的病变包括轻度腰椎滑脱、腰椎椎管狭窄或退行性椎间盘突出,但对于中度及以上的滑脱、多节段退变性侧弯或严重不稳定病变患者,BPSF可能仍是更优选择。 2.4.3 适应证与禁忌证 在腰椎退行性疾病的治疗中,由于微创的特性、较短的手术时间以及优良的临床效果,腰椎融合术联合UPSF已经逐渐开始在临床上普及。该术式对病例选择的要求较为严格,合理掌握其适应证与禁忌证对于手术的成功实施以及患者的长期预后具有关键意义(表2)。"
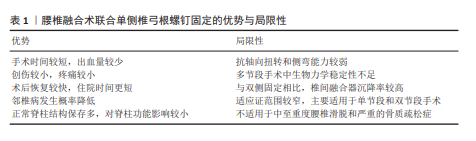
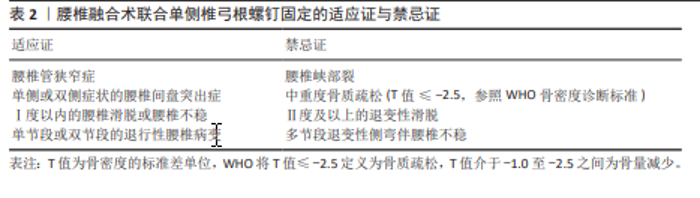
根据现有研究及临床经验,腰椎融合术联合UPSF适用于以下情况:单侧或双侧症状的腰椎间盘突出症、腰椎管狭窄症、复发性腰椎间盘突出症、单节段或双节段的退行性腰椎病变,以及Ⅰ度以内的腰椎滑脱或腰椎不稳。在这些适应证中,患者的病变范围与脊柱稳定性要求相对有限,能够在UPSF提供的生物力学稳定性范围内实现理想的手术疗效及脊柱功能恢复。但对于中重度骨质疏松的患者(依据WHO标准:T值≤-2.5为骨质疏松,T值介于-1.0至-2.5为骨量减少)特别是T值≤-2.5的患者,由于椎体骨质量下降,UPSF可能无法提供足够的固定强度和融合效果,因此被视为禁忌证。对于存在腰椎峡部裂、Ⅱ度及以上退变性滑脱、多节段退变性侧弯伴腰椎不稳,以及对侧解剖结构缺损或不完整的患者,因固定强度不足,UPSF同样被视为禁忌证。 腰椎融合术联合UPSF在适应证范围内展现出诸多显著优势,包括减少术中失血量、降低术后疼痛及软组织损伤、缩短住院时间,并显著降低邻近节段退变的发生率。未来的研究应重点关注优化UPSF的生物力学设计,可结合微创技术(如机器人导航与全内镜技术)以进一步提升其精准性与安全性,也应通过多中心大样本的随机对照研究进一步验证其长期疗效及复杂病例中的应用潜力,促进该术式在腰椎退行性疾病治疗中的广泛应用,为患者提供更为个性化且高效的治疗方案。"

| [1] ZHANG K, XU H, DU L, et al. Application of self-anchored lateral lumbar interbody fusion in lumbar degenerative diseases. BMC Musculoskelet Disord. 2023;24(1):836. [2] BAO J, ZOU D, LI W. Characteristics of the DXA Measurements in Patients Undergoing Lumbar Fusion for Lumbar Degenerative Diseases: A Retrospective Analysis of Over 1000 Patients. Clin Interv Aging. 2021;16: 1131-1137. [3] PAASSILTA P, LOHINIVA J, GÖRING HH, et al. Identification of a novel common genetic risk factor for lumbar disk disease. Jama. 2001;285(14):1843-1849. [4] DORAISAMY R, RAMASWAMI K, SHANMUGAM J, et al. Genetic risk factors for lumbar disc disease. Clin Anat. 2021; 34(1):51-56. [5] RAVINDRA VM, SENGLAUB SS, RATTANI A, et al. Degenerative Lumbar Spine Disease: Estimating Global Incidence and Worldwide Volume. Global Spine J. 2018; 8(8):784-794. [6] BRINJIKJI W, DIEHN FE, JARVIK JG, et al. MRI Findings of Disc Degeneration are More Prevalent in Adults with Low Back Pain than in Asymptomatic Controls: A Systematic Review and Meta-Analysis. AJNR Am J Neuroradiol. 2015;36(12):2394-2399. [7] MATSUMOTO M, OKADA E, TOYAMA Y, et al. Tandem age-related lumbar and cervical intervertebral disc changes in asymptomatic subjects. Eur Spine J. 2013;22(4):708-713. [8] IŞIK HS, OKUTAN Ö, YILDIRIM T, et al. Comparison of Unilateral versus Bilateral Pedicle Screw Fixation in Transforaminal Lumbar Interbody Fusion for Single Level Lumbar Degenerative Diseases and Review of Literature. Turk Neurosurg. 2017. doi: 10.5137/1019-5149.JTN.20531-17.1. [9] MATTHEWS JH. Nonsurgical treatment of pain in lumbar spine stenosis. Am Fam Physician. 1999;59(2):280,283-284. [10] YOSHIHARA H, YONEOKA D. National trends in the surgical treatment for lumbar degenerative disc disease: United States, 2000 to 2009. Spine J. 2015;15(2):265-271. [11] WANG SK, LI YJ, WANG P, et al. Safety and benefit of ambulation within 24 hours in elderly patients undergoing lumbar fusion: propensity score matching study of 882 patients. Spine J. 2024;24(5):812-819. [12] 李振宙, 侯树勋. 腰椎退行性疾病微创外科治疗概况及展望[J]. 中国骨与关节杂志,2022,11(9):641-647. [13] SHAHI P, VAISHNAV AS, MAI E, et al. Practical answers to frequently asked questions in minimally invasive lumbar spine surgery. Spine J. 2023;23(1):54-63. [14] LIN GX, HE LR, NAN JN, et al. Comparing Outcomes of Banana-Shaped and Straight Cages in Transforaminal Lumbar Interbody Fusion for Lumbar Degenerative Diseases: A Systematic Review and Meta-Analysis. Neurospine. 2024;21(1):261-272. [15] TSUJIMOTO T, ITOGA R, KANAYAMA M, et al. Clinical outcomes of short-segment lumbar fusion in patients older than 85 years with a minimum 2-year follow-up. J Neurosurg Spine. 2023;39(1):40-46. [16] AMBATI DV, WRIGHT EK JR, LEHMAN RA JR, et al. Bilateral pedicle screw fixation provides superior biomechanical stability in transforaminal lumbar interbody fusion: a finite element study. Spine J. 2015;15(8): 1812-1822. [17] GU G, ZHANG H, FAN G, et al. Clinical and radiological outcomes of unilateral versus bilateral instrumentation in two-level degenerative lumbar diseases. Eur Spine J. 2015;24(8):1640-1648. [18] REN C, QIN R, SUN P, et al. Effectiveness and safety of unilateral pedicle screw fixation in transforaminal lumbar interbody fusion (TLIF): a systematic review and meta-analysis. Arch Orthop Trauma Surg. 2017; 137(4):441-450. [19] LIU F, FENG Z, ZHOU X, et al. Unilateral Versus Bilateral Pedicle Screw Fixation in Transforaminal Lumbar Interbody Fusion: A Monocentric Study of 215 Patients With a Minimum of 4-Year Follow-up. Clin Spine Surg. 2017;30(6):E776-E783. [20] CHEN HH, CHEUNG HH, WANG WK, et al. Biomechanical analysis of unilateral fixation with interbody cages. Spine (Phila Pa 1976). 2005;30(4):E92-96. [21] XIN Z, LI W. Unilateral versus bilateral pedicle screw fixation in short-segment lumbar spinal fusion: a meta-analysis of randomised controlled trials. Int Orthop. 2016;40(2):355-364. [22] YUAN C, CHEN K, ZHANG H, et al. Unilateral versus bilateral pedicle screw fixation in lumbar interbody fusion: a meta-analysis of complication and fusion rate. Clin Neurol Neurosurg. 2014;117:28-32. [23] DADA A, SAGGI S, AMBATI VS, et al. Evolution of the Minimally Invasive Surgery Transforaminal Lumbar Interbody Fusion: Where Are We Now? Neurosurgery. 2025; 96(3s):S33-S41. [24] 程志坚, 贺西京. 腰椎融合术选择策略及发展趋势[J]. 中国骨伤,2024,37(8):746-749. [25] LEONARDI M, SIMONETTI L, AGATI R. Neuroradiology of spine degenerative diseases. Best Pract Res Clin Rheumatol. 2002;16(1):59-87. [26] FROST BA, CAMARERO-ESPINOSA S, FOSTER EJ. Materials for the Spine: Anatomy, Problems, and Solutions. Materials (Basel). 2019;12(2):253. [27] JAUMARD NV, LEUNG J, GOKHALE AJ, et al. Relevant Anatomic and Morphological Measurements of the Rat Spine: Considerations for Rodent Models of Human Spine Trauma. Spine (Phila Pa 1976). 2015;40(20):E1084-1092. [28] MA Z, LIU X, ZHANG M, et al. Research Progress on the Role of Cartilage Endplate in Intervertebral Disc Degeneration. Cell Biochem Funct. 2024;42(7):e4118. [29] WIDMER J, CORNAZ F, SCHEIBLER G, et al. Biomechanical contribution of spinal structures to stability of the lumbar spine-novel biomechanical insights. Spine J. 2020; 20(10):1705-1716. [30] HEUER F, SCHMIDT H, KLEZL Z, et al. Stepwise reduction of functional spinal structures increase range of motion and change lordosis angle. J Biomech. 2007; 40(2):271-280. [31] BOSZCZYK BM, BOSZCZYK AA, PUTZ R. Comparative and functional anatomy of the mammalian lumbar spine. Anat Rec. 2001;264(2):157-168. [32] CRAWFORD RP, CANN CE, KEAVENY TM. Finite element models predict in vitro vertebral body compressive strength better than quantitative computed tomography. Bone. 2003;33(4):744-750. [33] SHAH JS, HAMPSON WG, JAYSON MI. The distribution of surface strain in the cadaveric lumbar spine. J Bone Joint Surg Br. 1978;60-b(2):246-251. [34] NYGAARD OP, MELLGREN SI. The function of sensory nerve fibers in lumbar radiculopathy. Use of quantitative sensory testing in the exploration of different populations of nerve fibers and dermatomes. Spine (Phila Pa 1976). 1998; 23(3):348-352; discussion 553. [35] LIVSHITS G, POPHAM M, MALKIN I, et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis. 2011;70(10):1740-1745. [36] SALO S, HURRI H, RIKKONEN T, et al. Association between severe lumbar disc degeneration and self-reported occupational physical loading. J Occup Health. 2022;64(1):e12316. [37] MAYER JE, IATRIDIS JC, CHAN D, et al. Genetic polymorphisms associated with intervertebral disc degeneration. Spine J. 2013;13(3):299-317. [38] ASHLEY JW, ENOMOTO-IWAMOTO M, SMITH LJ, et al. Intervertebral disc development and disease-related genetic polymorphisms. Genes Dis. 2016;3(3):171-177. [39] ECK JC, SHARAN A, RESNICK DK, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 6: discography for patient selection. J Neurosurg Spine. 2014; 21(1):37-41. [40] WATTERS WC 3RD, RESNICK DK, ECK JC, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 13: injection therapies, low-back pain, and lumbar fusion. J Neurosurg Spine. 2014;21(1):79-90. [41] DIVI SN, SCHROEDER GD, GOYAL DKC, et al. Fusion technique does not affect short-term patient-reported outcomes for lumbar degenerative disease. Spine J. 2019;19(12): 1960-1968. [42] HIBBS RA. An operation for progressive spinal deformities: a preliminary report of three cases from the service of the orthopaedic hospital 1911. Clin Orthop Relat Res. 2007;460:17-20. [43] CLOWARD RB. The treatment of ruptured lumbar intervertebral disc by vertebral body fusion. III. Method of use of banked bone. Ann Surg. 1952;136(6):987-992. [44] HARMS J, ROLINGER H. A one-stager procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion (author’s transl). Z Orthop Ihre Grenzgeb. 1982;120(3): 343-347. [45] DE KUNDER SL, VAN KUIJK SMJ, RIJKERS K, et al. Transforaminal lumbar interbody fusion (TLIF) versus posterior lumbar interbody fusion (PLIF) in lumbar spondylolisthesis: a systematic review and meta-analysis. Spine J. 2017;17(11):1712-1721. [46] LAN T, HU SY, ZHANG YT, et al. Comparison Between Posterior Lumbar Interbody Fusion and Transforaminal Lumbar Interbody Fusion for the Treatment of Lumbar Degenerative Diseases: A Systematic Review and Meta-Analysis. World Neurosurg. 2018; 112:86-93. [47] KUNZE B, DRASSECK T, KLUBA T. Posterior and transforaminal lumbar interbody fusion (PLIF/TLIF) for the treatment of localised segment degeneration of lumbar spine. Z Orthop Unfall. 2011;149(3):312-316. [48] FOLEY KT, GUPTA SK. Percutaneous pedicle screw fixation of the lumbar spine: preliminary clinical results. J Neurosurg. 2002;97(1 Suppl):7-12. [49] YOUN MS, SHIN JK, GOH TS, et al. Full endoscopic lumbar interbody fusion (FELIF): technical note. Eur Spine J. 2018;27(8): 1949-1955. [50] YIN P, ZHANG Y, PAN A, et al. The feasibility for a novel minimally invasive surgery-percutaneous endoscopic transforaminal lumbar interbody fusion (PE-TLIF) for the treatment of lumbar degenerative diseases: a cadaveric experiment. J Orthop Surg Res. 2020;15(1):387. [51] ZHU L, CAI T, SHAN Y, et al. Comparison of Clinical Outcomes and Complications Between Percutaneous Endoscopic and Minimally Invasive Transforaminal Lumbar Interbody Fusion for Degenerative Lumbar Disease: A Systematic Review and Meta-Analysis. Pain Physician. 2021;24(6):441-452. [52] KAMSON S, LU D, SAMPSON PD, et al. Full-Endoscopic Lumbar Fusion Outcomes in Patients with Minimal Deformities: A Retrospective Study of Data Collected Between 2011 and 2015. Pain Physician. 2019;22(1) 75-88. [53] 吴泽宣, 王涛, 雷志刚, 等. 两种固定方式在单侧双通道脊柱内镜技术下单节段椎间融合固定术中的对照研究[J]. 中国骨伤,2024,37(12):1158-1163. [54] CHENG X, ZHANG K, SUN X, et al. Unilateral versus bilateral pedicle screw fixation with transforaminal lumbar interbody fusion for treatment of lumbar foraminal stenosis. Spine J. 2022;22(10):1687-1693. [55] KABINS MB, WEINSTEIN JN, SPRATT KF, et al. Isolated L4-L5 fusions using the variable screw placement system: unilateral versus bilateral. J Spinal Disord. 1992;5(1):39-49. [56] 吴陈, 龙浩, 敖翔, 等. 单侧与双侧椎弓钉固定腰椎后路椎体间融合比较[J]. 中国矫形外科杂志,2024,32(7):608-613. [57] YANG X, WANG H, ZHAO Q, et al. A comparison of unilateral and bilateral pedicle screw fixation combined with transforaminal lumbar interbody fusion for lumbar degenerative diseases. Chin Med J (Engl). 2014;127(20):3592-3596. [58] XUE H, TU Y, CAI M. Comparison of unilateral versus bilateral instrumented transforaminal lumbar interbody fusion in degenerative lumbar diseases. Spine J. 2012;12(3): 209-215. [59] KIM HJ, KANG KT, CHANG BS, et al. Biomechanical analysis of fusion segment rigidity upon stress at both the fusion and adjacent segments: a comparison between unilateral and bilateral pedicle screw fixation. Yonsei Med J. 2014;55(5): 1386-1394. [60] 邹海波, 绳厚福, 李中实, 等. 微创TLIF单侧或双侧固定治疗腰椎退行性疾病的临床疗效[J]. 中国脊柱脊髓杂志,2013, 23(12):1086-1091. [61] SAINI S, MOGER NM, KUMAR M, et al. Biomechanical analysis of Instrumented decompression and Interbody fusion procedures in Lumbar spine: a finite element analysis study. Med Biol Eng Comput. 2023;61(7):1875-1886. [62] YüCESOY K, YüKSEL KZ, BAEK S, et al. Biomechanics of unilateral compared with bilateral lumbar pedicle screw fixation for stabilization of unilateral vertebral disease. J Neurosurg Spine. 2008;8(1):44-51. [63] LI J, XIAO H, ZHU Q, et al. Novel pedicle screw and plate system provides superior stability in unilateral fixation for minimally invasive transforaminal lumbar interbody fusion: an in vitro biomechanical study. PLoS One. 2015;10(3):e0123134. [64] DU L, SUN XJ, ZHOU TJ, et al. The role of cage height on the flexibility and load sharing of lumbar spine after lumbar interbody fusion with unilateral and bilateral instrumentation: a biomechanical study. BMC Musculoskelet Disord. 2017;18(1): 474. [65] SUK KS, LEE HM, KIM NH, et al. Unilateral versus bilateral pedicle screw fixation in lumbar spinal fusion. Spine (Phila Pa 1976). 2000;25(14):1843-1847. [66] XIAO SW, JIANG H, YANG LJ, et al. Comparison of unilateral versus bilateral pedicle screw fixation with cage fusion in degenerative lumbar diseases: a meta-analysis. Eur Spine J. 2015;24(4):764-774. [67] VILLAVICENCIO AT, SERXNER BJ, MASON A, et al. Unilateral and bilateral pedicle screw fixation in transforaminal lumbar interbody fusion: radiographic and clinical analysis. World Neurosurg. 2015;83(4):553-559. [68] BERINGER WF, MOBASSER JP. Unilateral pedicle screw instrumentation for minimally invasive transforaminal lumbar interbody fusion. Neurosurg Focus. 2006;20(3):E4. [69] GIORGI H, PREBET R, ANDRIANTSIMIAVONA R, et al. Minimally invasive transforaminal lumbar interbody fusion with unilateral pedicle screw fixation (UNILIF): morbidity, clinical and radiological 2-year outcomes of a 66-patient prospective series. Eur Spine J. 2018;27(8):1933-1939. [70] CHOI UY, PARK JY, KIM KH, et al. Unilateral versus bilateral percutaneous pedicle screw fixation in minimally invasive transforaminal lumbar interbody fusion. Neurosurg Focus. 2013;35(2):E11. |
| [1] | Zheng Wangyang, Fei Ji, Yang Di, Zhao Lang, Wang Lingli, Liu Peng, Li Haiyang. Finite element analysis of the force changes of the supraspinatus tendon and glenohumeral joint during the abduction and flexion of the humerus [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2199-2207. |
| [2] | Cai Qirui, Dai Xiaowei, Zheng Xiaobin, Jian Sili, Lu Shaoping, Liu Texi, Liu Guoke, Lin Yuanfang. Mechanical effects of Long’s traction orthopedic method on cervical functional units: quantitative analysis of biomechanical model of head and neck [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2208-2216. |
| [3] | Rao Jingcheng, Li Yuwan, Zheng Hongbing, Xu Zhi, Zhu Aixiang, Shi Ce, Wang Bing, Yang Chun, Kong Xiangru, Zhu Dawei. Biomechanical differences between the new proximal femoral stable intramedullary nail and traditional intramedullary nail#br# [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2217-2225. |
| [4] | Chen Long, Wang Xiaozhen, Xi Jintao, Lu Qilin. Biomechanical performance of short-segment screw fixation combined with expandable polyetheretherketone vertebral body replacement in osteoporotic vertebrae [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2226-2235. |
| [5] | Yan Xiangning, Chen Lei, Chen Yonghuan, Wang Chao, Li Xiaosheng. Influence of different depths and loads on knee joint mechanics and peripheral muscle force characteristics during squatting [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2236-2247. |
| [6] | Zeng Xuan, Weng Rui, Ye Shicheng, Tang Jiadong, Mo Ling, Li Wenchao. Two lumbar rotary manipulation techniques in treating lumbar disc herniation: a finite element analysis of biomechanical differences [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2153-2161. |
| [7] | Wu Hongxu, Liu Xuanyu, Wang Taoyu, Wang Shiyao, Cheng Jingyi, Zhang Mingwen, Zhang Yinxia, Liu Zhihua, Wang Xiaojie. Finite element simulation of scoliosis with muscle unit introduction: verification of correction effect under bidirectional load [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2172-2181. |
| [8] | Liu Jiafu, Ren Ruxia, Liao Zhouwei, Zhou Xiali, Wu Yihong, Zhang Shaoqun. Three-dimensional finite element analysis of cervical spine biomechanical characteristics in a rat model of cervical vertigo [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2182-2190. |
| [9] | Zhou Daobin, Wang Kehao, Xie Yang, Ning Rende. Biomechanical characteristics of volar locking plate only versus combined dorsal mini-plate fixation of distal radius fractures with dorsal ulnar fragment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2255-2261. |
| [10] | Zhang Nan, Meng Qinghua, Bao Chunyu. Characteristics and clinical application of ankle joint finite element models [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2343-2349. |
| [11] | Wen Fayan, Li Yan, Qiang Tianming, Yang Chen, Shen Linming, Li Yadong, Liu Yongming. Unilateral biportal endoscopic technology for treatment of lumbar degenerative diseases: global research status and changing trends [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2380-2390. |
| [12] | Zhong Caihong, Xiao Xiaoge, Li Ming, Lin Jianhong, Hong Jing. Biomechanical mechanism of sports-related patellar tendinitis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1417-1423. |
| [13] | Yu Xinlin, Chen Huiyu, Wang Yingying, Guo Weizhong, Feng Bin Lin Chengshou, Lin Wang. Finite element analysis of internal fixation with new retrograde intramedullary nail on lateral femur condyle for distal type A2 femur fractures [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 546-552. |
| [14] | Zhao Jingang, Liu Liping, Chen Jianwei, . Finite element analysis comparing lumbar fusion and artificial intervertebral disc replacement [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 553-560. |
| [15] | Ma Jingbo, Yang Guangnan, Liu Jiang, Jiang Qiang, Zhang Hanshuo, Han Jiaheng, Ding Yu. Endoscopic lumbar canal decompression for upper lumbar spinal stenosis: a comparison of biomechanical stability of three surgical models [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 577-585. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||