Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (23): 5982-5991.doi: 10.12307/2026.366
Previous Articles Next Articles
Mitophagy regulates osteoclasts: a new perspective for osteoporosis treatment
Gao Jiabin1, Li Tianqi1, Xu Kun1, Zhu Hanmin1, Zhou Xi2, Li Wei1
- 1Medicine College, Shaoxing University, Shaoxing 312099, Zhejiang Province, China; 2Basic Medical College, Hubei University of Arts and Sciences, Xiangyang 441053, Hubei Province, China
-
Received:2025-06-30Accepted:2025-09-01Online:2026-08-18Published:2025-12-31 -
Contact:Li Wei, PhD, Associate professor, Medicine College, Shaoxing University, Shaoxing 312099, Zhejiang Province, China -
About author:Gao Jiabin, Medicine College, Shaoxing University, Shaoxing 312099, Zhejiang Province, China -
Supported by:National Natural Science Foundation of China (Youth Program), No. 82205234 (to LW); Hubei Provincial Natural Science Foundation Joint Fund Project, No. 2024AFD049 (to ZX); Xiangyang Municipal Science and Technology Bureau Key Project, No. 2022YL07A (to LW); Zhejiang Medical Association Clinical Medicine Special Fund Project, No. 2023ZYC-A182 (to LW); Shaoxing University Key Research Project, No. 2024LG011 (to ZHM); Shaoxing University Research Start-up Fund Project (to LW); Shaoxing Municipal Basic Public Welfare Special Project, No. 2025A14022 (to LW)
CLC Number:
Cite this article
Gao Jiabin, Li Tianqi, Xu Kun, Zhu Hanmin, Zhou Xi, Li Wei. Mitophagy regulates osteoclasts: a new perspective for osteoporosis treatment[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 5982-5991.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

2.1 线粒体自噬在破骨细胞领域应用发展的关键时间点及重要内容 线粒体自噬这一概念最早由LEMASTERS于2005年正式提出。2016年,日本科学家大隅良典因“发现细胞自噬机制”获得了诺贝尔生理学或医学奖,此后关于自噬及线粒体自噬的研究在各个领域掀起了热潮。在骨代谢领域,关于自噬和线粒体自噬的研究是一种细胞内降解机制,线粒体自噬是自噬过程中的一个特定类型,专门负责清除受损或多余的线粒体。在骨动态平衡的维持过程中,线粒体自噬或自噬的研究大多数都靶向了成骨细胞,有关破骨细胞诱发的骨吸收过程研究相对较少。随着研究的深入,破骨细胞自噬相关机制也逐渐被揭示,主要的信号通路包括缺氧诱导因子 1、GTP酶偶联受体激酶 2、动力学相关蛋白、隔离素1、Bec1in1基因、信号转导和转录激活因子3、核因子kB受体激活因子配体/p38丝裂原活化蛋白激酶、哺乳动物雷帕霉素靶蛋白等。线粒体自噬在破骨细胞领域应用发展的关键时间点及重要内容[7-16],见表1。 2.2 线粒体自噬 2.2.1 线粒体自噬机制 线粒体自噬是真核细胞中一种关键的线粒体质量控制机制,主要功能是选择性地清除损伤或功能紊乱的线粒体。当细胞面临活性氧压力、营养匮乏、衰老或其他形式的应激时,线粒体会发生去极化损伤[17],导致线粒体DNA突变累积以及线粒体外膜电位下降。为了维护线粒体和细胞的整体稳态,防止受损线粒体对细胞造成进一步损伤,细胞会启动一个特定过程,即通过自噬体识别并包裹这些受损的线粒体,形成线粒体自噬体;随后,线粒体自噬体与溶酶体融合,完成线粒体自噬的全过程,这一过程对于确保细胞在营养不足或外部刺激条件下仍能保持正常的生理活动极为重要。然而,线粒体自噬异常可能导致线粒体功能障碍,进而引起细胞内稳态丧失,甚至诱发细胞凋亡,这表明适当的线粒体自噬对于保持线粒体网络的功能完整性和细胞生存有重要意义。因此,线粒体自噬是一个复杂的“双刃剑”,它既可能带来积极影响,也可能带来潜在风险。 2.2.2 线粒体自噬途径 研究发现,线粒体自噬主要通过2条关键途径进行:同源性磷酸酶张力蛋白诱导的激酶/E3泛素连接酶途径和受体介导的线粒体自噬途径。其中,受体介导的线粒体自噬途径主要包括含FUN14域蛋白1、BNIP3L/NIX和B细胞白血病2样凋亡蛋白13途径[18-19]。此外,线粒体自噬还有受到自噬相关因子的调控,例如巨噬细胞迁移因子1、p66SHC等[20-21]。该小节将重点阐述上述线粒体自噬途径的调控机制。 (1)同源性磷酸酶张力蛋白诱导的激酶/E3泛素连接酶途径:线粒体不仅是细胞的能量代谢中心,负责为细胞的生命活动提供必要的能量,同时也是细胞内活性氧的主要产生地[22-23]。ZHAO等[24]的研究揭示,通过激活由同源性磷酸酶张力蛋白诱导的激酶/E3泛素连接酶介导的线粒体自噬途径,可以有效降低细胞内活性氧水平,从而抑制由衰老引起的细胞凋亡。在正常生理状态下,新合成的同源性磷酸酶张力蛋白诱导的激酶蛋白在转移到线粒体内膜的过程中被蛋白酶剪切,失去它特定结构域后被释放到细胞质中,并被泛素-蛋白酶体系统降解。然而在受损的线粒体中,由于线粒体膜电位的下降,同源性磷酸酶张力蛋白诱导的激酶无法完成正常转运过程,而是积聚在线粒体的外膜上招募并激活E3泛素连接酶蛋白,E3泛素连接酶蛋白一旦被激活便会催化线粒体外膜上的蛋白质泛素化,从而标记了线粒体作为自噬的靶点,促使线粒体被自噬体包裹并与溶酶体融合,最终实现对受损线粒体的降解和清除[25] (图3)。因此,同源性磷酸酶张力蛋白诱导的激酶/E3泛素连接酶信号通路在调节线粒体自噬过程中扮演了一个核心且成熟的调控角色。"
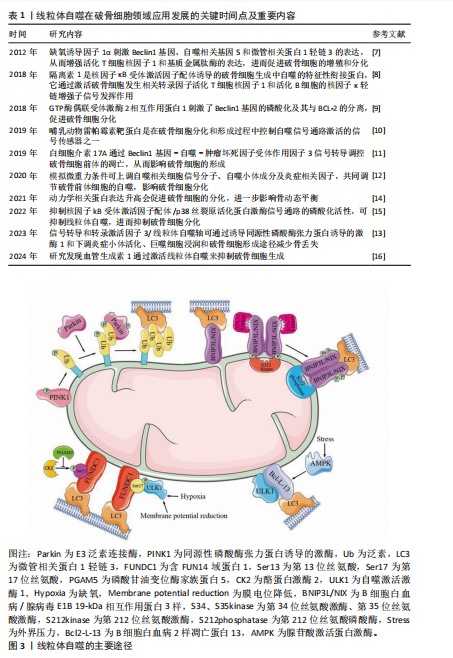
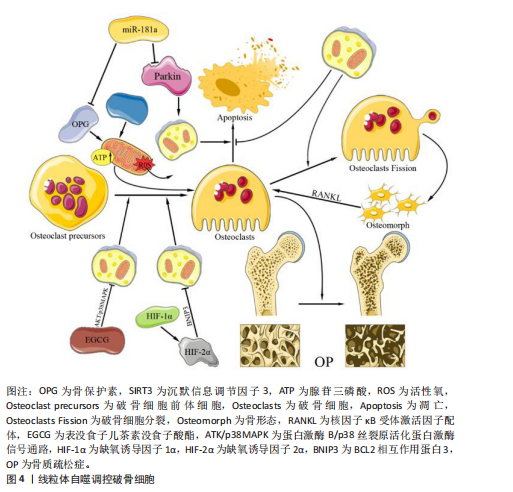
(2)受体介导的线粒体自噬途径 含FUN14域蛋白1途径:相关研究表明,含FUN14域蛋白1的磷酸化水平与许多疾病的发生密切相关,尤其是心血管疾病和帕金森症[26-28],这提示含FUN14域蛋白1所调控的线粒体自噬对细胞维持正常生理功能有重要意义。罗以楠等[29]提出在缺氧环境下或线粒体膜电位降低时,自噬激活激酶1等UNC-51家族激酶会增强含FUN14域蛋白1的Ser17磷酸化,从而诱导线粒体自噬的发生[30]。正常情况下,酪蛋白激酶2会与磷酸化含FUN14域蛋白1上的Ser13特异性结合,使其失活,从而抑制由含FUN14域蛋白1途径介导的线粒体自噬。而磷酸甘油变位酶家族蛋白5可以使含FUN14域蛋白1的Ser13发生去磷酸化,从而抑制酪蛋白激酶与磷酸化含FUN14域蛋白1的Ser13的特异性结合[31]。综上所述,含FUN14域蛋白1途径介导的细胞线粒体自噬受到多种因素的精细调控,但核心机制均是通过FUN14域蛋白1的微管相关蛋白1轻链3相互作用区与微管相关蛋白1轻链3介导的自噬体结合,从而开启对特定线粒体识别并结合的自噬过程。 BNIP3L/NIX途径:目前研究最多的线粒体自噬受体之一是BNIP3L/NIX途径,该途径对多种细胞终末分化过程中健康线粒体的程序性去除很重要[18]。在线粒体自噬信号诱导后,BNIP3L/NIX通过其N端的微管相关蛋白1轻链3相互作用区与自噬体的微管相关蛋白1轻链3结合,从而促进线粒体自噬。但在特定信号诱导下,Ser212激酶会将磷酸化的BNIP3L/NIX单体在C端去磷酸化,延伸至线粒体膜间隙,形成更稳定的BNIP3L/NIX二聚体。同时,Ser34、Ser35激酶会将微管相关蛋白1轻链3相互作用区上的Ser34和Ser35磷酸化,这种双微管相关蛋白1轻链3相互作用区磷酸化会增强线粒体上自噬体的募集能力。MARINKOVI?等[32]的研究表明,微管相关蛋白1轻链3相互作用区磷酸化和受体二聚化的联合机制是BNIP3L/NIX依赖性线粒体自噬正常启动和进展的必要条件。BNIP3L/NIX磷酸化和二聚化之间存在相互作用,在这种双重磷酸化激活后BNIP3L/NIX形成稳定的同源二聚体,相较于其单体形式更能有效募集自噬体[31](图3)。综上所述,由BNIP3L/NIX信号通路介导的线粒体自噬在维持线粒体质量过程中发挥着不可替代的作用,未来研究可进一步探寻BNIP3L/NIX二聚体形成的诱发条件以提高二聚体的形成水平,诱导产生高效的线粒体自噬。 B细胞白血病2样凋亡蛋白13途径:自噬相关蛋白32蛋白作为酿酒酵母菌线粒体的一种外膜蛋白,早在2009年就被发现为诱导线粒体自噬的重要因子[33-35]。2015年,MURAKAWA及其团队[36]通过蛋白质序列比对确定哺乳动物体内的B细胞白血病2样凋亡蛋白13是自噬相关蛋白32的同源物,B细胞白血病2样凋亡蛋白13在自噬相关蛋白32缺陷型酵母细胞中诱导线粒体裂变和自噬,发挥着补偿酵母中自噬相关蛋白32功能的能力。在被诱导自噬的线粒体中,B细胞白血病2样凋亡蛋白13能够募集并结合自噬激活激酶1复合物,而后自噬激活激酶1通过B细胞白血病2样凋亡蛋白13复合物中的微管相关蛋白1轻链3相互作用区基序与微管相关蛋白1轻链3B相互作用,诱导自噬体的形成,促进线粒体自噬的发生,这一发现揭示了B细胞白血病2样凋亡蛋白13在介导哺乳动物细胞的线粒体裂变和自噬的关键作用[37]。在最新的研究中,MURAKAWA等[38]进一步阐明B细胞白血病2样凋亡蛋白13的调控机制,发现腺苷酸激活蛋白激酶催化亚单位α-2是负责B细胞白血病2样凋亡蛋白13在Ser272位点磷酸化的关键激酶,在线粒体因压力超负荷而损伤后,腺苷酸激活蛋白激酶将被激活并通过磷酸化 B细胞白血病2样凋亡蛋白13的Ser272位点激活线粒体自噬,并降解受损的线粒体(图3)。然而,B细胞白血病2样凋亡蛋白13途径在破骨细胞线粒体中的潜在机制尚未被揭示,无法对解破骨细胞中线粒体自噬的调控机制有全面了解。 (3)其他自噬受体介导的途径:除了上述主要线粒体自噬途径,还存在许多由其他自噬因子调控的线粒体自噬。巨噬细胞迁移因子1在以往的研究中总是作为一种炎症和神经内分泌递质,能够促进免疫细胞的活化和其他促炎性细胞因子的产生,在巨噬细胞的迁移中发挥重要作用[39]。WANG及其团队[40]发现,巨噬细胞迁移因子1的缺乏加剧了活性氧的积累、线粒体功能障碍和细胞在过载机械压缩下的衰老。XUE等[11]的研究发现,巨噬细胞迁移因子1升高后会直接与同源性磷酸酶张力蛋白诱导的激酶结合,从而破坏激酶与E3泛素连接酶的相互作用,进而抑制线粒体自噬的启动。然而,巨噬细胞迁移因子1和同源性磷酸酶张力蛋白诱导的激酶在分子结构中的蛋白质相互作用尚未阐明,因此,该结合位点在未来可作为调控线粒体自噬的研究靶点。 p66SHC作为是一种负性寿命调节因子,已被证实在线粒体中发挥促氧化作用[41]。ONNIS等[21]的实验进一步揭示了p66SHC在B细胞中与自噬及线粒体自噬有关,从而进一步影响细胞存活。p66SHC通过2个途径进行对线粒体自噬的介导:破坏线粒体的完整性,诱导自噬并引发线粒体的降解;发挥线粒体自噬受体的作用,介导受损线粒体被自噬体包裹并最终被溶酶体识别降解。p66SHC通过其细胞色素C结合域破坏线粒体功能,导致ATP生成减少,从而激活酪蛋白激酶2并增强自噬通量。同时,p66SHC作为受体与微管相关蛋白1轻链3-Ⅱ特异性结合并将吞噬泡膜带到线粒体,诱发自噬的启动。虽然已经明确p66SHC可以作为受体诱导线粒体自噬,但介导p66SHC与微管相关蛋白1轻链3-Ⅱ结合的上游因子和p66SHC与线粒体结合方式尚不明确,这些问题的深入研究将为进一步理解线粒体自噬的分子机制提供重要线索。 2.2.3 当前线粒体自噬研究的局限性 线粒体自噬涉及多种信号通路和分子机制,但其调控网络尚未完全清晰,如E3泛素连接酶诱导的线粒体自噬和受体依赖性线粒体自噬之间的相互作用仍需进一步探究。当前的线粒体自噬诱导剂主要以线粒体毒素和基于信号通路的诱导剂为主,这些药物在研究和临床中有着重要意义,但其中大部分药物研究仍处于初步阶段,大部分数据仅来源于细胞实验和动物实验,需要进一步探究药物的作用机制和临床应用前景。同时,由于线粒体自噬的“双刃剑”特性,过高的药物剂量将引起一定的负面效果,所以,寻找药物的最佳剂量应聚焦于如何精准调控线粒体自噬水平,是线粒体自噬药物从理论走向临床途中必须克服的挑战之一。 2.3 线粒体自噬对破骨细胞的影响 骨骼的稳定性通过正常水平的骨代谢和骨稳态来维持[42],在这一过程中,破骨细胞通过分泌酸与降解酶介导的骨吸收在维持骨重建或骨改建中起重要作用[43]。近年来,大量研究揭示了自噬和线粒体自噬与骨代谢有着错综复杂的联系[44],然而破骨细胞作为骨代谢中重要的细胞之一,却鲜有相关文献叙述线粒体自噬对破骨细胞的影响,以下将归纳并阐述线粒体自噬对破骨细胞的影响以及作用机制。 2.3.1 线粒体自噬维持破骨细胞活性氧内稳态 线粒体作为活性氧的主要生产场所之一,它累积的活性氧不仅会引发细胞损伤,还可能引发线粒体功能障碍。研究表明,活性氧对破骨细胞的作用具有浓度依赖性,适宜浓度的活性氧促进破骨细胞分化[45],而高浓度的活性氧会导致破骨细胞凋亡;此外,高浓度的活性氧还可作为自噬信号调控线粒体自噬过程,清除受损的线粒体,从而维持破骨细胞内线粒体数量与质量的平衡[46]。相关研究证实,骨保护素能够降低破骨细胞中活性氧水平[47]。当细胞受到过高活性氧刺激时会启动自噬过程,以清除受损的线粒体,维持破骨细胞正常功能和稳态。 缺氧诱导因子是一种细胞氧传感和调节的核心转录因子,在缺氧条件下它与线粒体自噬之间存在密切联系[48]。HONG等[49]研究表明,BCL2相互作用蛋白3受到缺氧诱导因子的调节并影响细胞的存活,该机制为缺氧诱导因子提高细胞内BCL2相互作用蛋白3的表达,促进线粒体自噬,降低细胞内活性氧水平,维持线粒体功能的完整性。然而,在同源性磷酸酶张力蛋白诱导的激酶敲除小鼠体内,破骨细胞表现为线粒体自噬缺陷、活性氧积累增加,导致分化异常和过度凋亡[50]。因此,如果线粒体内活性氧的水平过高,导致破骨细胞内功能障碍的线粒体无法因自噬被清除,最终可能会破坏骨稳态,进而引发骨质疏松症的发生(图4)。 2.3.2 线粒体自噬调节破骨细胞能量代谢 沉默信息调节因子3作为一种依赖烟酰胺腺嘌呤二核苷酸(NAD+)去乙酰化酶,它在细胞内具有清除活性氧的功能,并通过去乙酰化调控细胞的生理状态[51-52]。此外,沉默信息调节因子3还可以调控线粒体凋亡、代谢及氧化应激等过程,它的表达水平增加可减轻骨量的流失[53]。 RICHARDSON等[54]通过将异性敲除小鼠骨髓谱系中的沉默信息调节因子3基因建立了相关小鼠模型,发现沉默信息调节因子3能够以一种同源性磷酸酶张力蛋白诱导的激酶非依赖性方式刺激破骨细胞中的线粒体质量,增强线粒体活性,从而维持破骨细胞线粒体能量代谢高效进行。而GUO等[55]的研究发现,沉默信息调节因子3的表达降低会诱发线粒体功能障碍,并抑制由同源性磷酸酶张力蛋白诱导的激酶/E3泛素连接酶通路介导的线粒体自噬,进而导致线粒体内能量代谢紊乱,ATP产生减少。因此,寻找高效的沉默信息调节因子3激动剂以减少破骨细胞线粒体内ATP产生,对衰老骨骼具有保护作用,可以为骨质疏松相关疾病提供了新的视角和治疗靶点(图4)。"
| [1] LESLIE WD, BURRELL S, MORIN SN. Fracture Risk Assessment in the 2023 Osteoporosis Canada Guideline. Can Assoc Radiol J. 2025;76(3):508-518. [2] KAHWATI LC, KISTLER CE, BOOTH G, et al. Screening for osteoporosis to prevent fractures: a systematic evidence review for the US Preventive Services Task Force. JAMA. 2025;333(6):509-531. [3] WANG J, SHU B, TANG DZ, et al. The prevalence of osteoporosis in China, a community based cohort study of osteoporosis. Front Public Health. 2023;11:1084005. [4] SUN Z, MA Z, CAO W, et al. Calcium-mediated mitochondrial fission and mitophagy drive glycolysis to facilitate arterivirus proliferation. PLoS Pathog. 2025;21(1):e1012872. [5] GAO DL, LIN MR, GE N, et al. From macroautophagy to mitophagy: Unveiling the hidden role of mitophagy in gastrointestinal disorders. World J Gastroenterol. 2024;30(23):2934-2946. [6] ZHANG K, LI Q, ZHANG Y, et al. Targeting Mitophagy as a Potential Therapeutic Approach for Age‐Related Bone Diseases. Advanced Therapeutics. 2024;7(7):2400078. [7] ZHAO Y, CHEN G, ZHANG W, et al. Autophagy regulates hypoxia‐induced osteoclastogenesis through the HIF‐1α/BNIP3 signaling pathway. J Cell Physiol. 2012;227(2):639-648. [8] ZACH F, POLZER F, MUELLER A, et al. p62/sequestosome 1 deficiency accelerates osteoclastogenesis in vitro and leads to Paget’s disease–like bone phenotypes in mice. J Biol Chem. 2018;293(24): 9530-9541. [9] ZHAO SJ, KONG FQ, CAI W, et al. GIT1 contributes to autophagy in osteoclast through disruption of the binding of Beclin1 and Bcl2 under starvation condition. Cell Death Dis. 2018;9(12):1195. [10] TONG X, GU J, SONG R, et al. Osteoprotegerin inhibit osteoclast differentiation and bone resorption by enhancing autophagy via AMPK/mTOR/p70S6K signaling pathway in vitro. J Cell Biochem. 2019;120(2):1630-1642. [11] XUE Y, LIANG Z, FU X, et al. IL-17A modulates osteoclast precursors’ apoptosis through autophagy-TRAF3 signaling during osteoclastogenesis. Biochem Biophys Res Commun. 2019;508(4):1088-1092. [12] WU CH, OU CH, YEN IC, et al. 4-Acetylantroquinonol B inhibits osteoclastogenesis by inhibiting the autophagy pathway in a simulated microgravity model. Int J Mol Sci. 2020;21(18):6971. [13] ZHU L, WANG Z, SUN X, et al. STAT3/mitophagy axis coordinates macrophage NLRP3 inflammasome activation and inflammatory bone loss. J Bone Miner Res. 2023;38(2):335-353. [14] JEONG S, SEONG JH, KANG JH, et al. Dynamin‐related protein 1 positively regulates osteoclast differentiation and bone loss. FEBS Lett. 2021;595(1):58-67. [15] SARKAR J, DAS M, HOWLADER MSI, et al. Epigallocatechin-3-gallate inhibits osteoclastic differentiation by modulating mitophagy and mitochondrial functions. Cell Death Dis. 2022;13(10):908. [16] YIN J, LAI P, ZHU L, et al. Angiopoietin 1 Relieves Osteolysis by Promoting Macrophage Mitophagy Through the TBK1-SQSTM1 Pathway to Inhibit AIM2 Inflammasome-Mediated Pyroptosis. Appl Biochem Biotechnol. 2024;196(11):7908-7927. [17] NAKATOGAWA H. Mechanisms governing autophagosome biogenesis. Nat Rev Mol Cell Biol. 2020;21(8):439-458. [18] MARINKOVIĆ M, NOVAK I. A brief overview of BNIP3L/NIX receptor‐mediated mitophagy. FEBS Open Bio. 2021;11(12):3230-3236. [19] D’ARCY MS. Mitophagy in health and disease. Molecular mechanisms, regulatory pathways, and therapeutic implications. Apoptosis. 2024;29(9):1415-1428. [20] LI T, QU J, HU C, et al. Macrophage migration inhibitory factor (MIF) suppresses mitophagy through disturbing the protein interaction of PINK1-Parkin in sepsis-associated acute kidney injury. Cell Death Dis. 2024;15(7):473. [21] ONNIS A, CIANFANELLI V, CASSIOLI C, et al. The pro-oxidant adaptor p66SHC promotes B cell mitophagy by disrupting mitochondrial integrity and recruiting LC3-II. Autophagy. 2018;14(12):2117-2138. [22] 唐恒芳.维生素K2在骨代谢及线粒体损伤修复中的作用研究[D].合肥:中国科学技术大学,2023. [23] SHADEL GS, HORVATH TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163(3):560-569. [24] ZHAO H, ZHANG Y, REN Y, et al. PINK1/Parkin-Mediated Mitophagy Ameliorates Mitochondrial Dysfunction in Lacrimal Gland Acinar Cells During Aging. Invest Ophthalmol Vis Sci. 2024; 65(13):12. [25] 沈华星. PINK1/Parkin信号通路调控成骨细胞分化及机制研究[D].上海:上海大学, 2023. [26] 张沛文,张醒醒,梁伦敏.运动介导线粒体自噬在心血管疾病中的作用[J].当代体育科技,2022,12(36):18-21. [27] 吕毓虎,程林,张沛文,等.UNDC1介导线粒体自噬参与运动预适应心肌保护的作用机制[C]//广州体育学院,中国体育科学学会运动生理生化分会,中国体育科学学会运动医学分会.2022年第七届广州运动与健康国际学术研讨会论文集.广西师范大学体育与健康学院,2022:304-305. [28] 张泰铭.FUNDC1通过促进线粒体自噬调控缺氧状态下帕金森病的作用及机制研究[D].沈阳:中国医科大学,2023. [29] 罗以楠,邱俏檬,卢中秋,等.线粒体自噬中受体蛋白FUNDC1研究进展[J].医学研究生学报,2020, 33(5):537-542. [30] ZHU Y, GU Z, SHI J, et al. Vaspin Attenuates Atrial Abnormalities by Promoting ULK1/FUNDC1‐Mediated Mitophagy. Oxid Med Cell Longev. 2022;2022(1):3187463. [31] 张凤娟,杨薇.BNIP3L/NIX介导线粒体自噬对缺血性脑卒中作用的研究进展[J].中风与神经疾病杂志,2022,39(10):949-951. [32] MARINKOVIĆ M, ŠPRUNG M, NOVAK I. Dimerization of mitophagy receptor BNIP3L/NIX is essential for recruitment of autophagic machinery. Autophagy. 2021; 17(5):1232-1243. [33] KANKI T, WANG K, CAO Y, et al. Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev Cell. 2009;17(1):98-109. [34] XIA X, KATZENELL S, REINHART EF, et al. A pseudo-receiver domain in Atg32 is required for mitophagy. Autophagy. 2018;14(9): 1620-1628. [35] KANKI T, KLIONSKY DJ. Atg32 is a tag for mitochondria degradation in yeast. Autophagy. 2009;5(8):1201-1202. [36] MURAKAWA T, YAMAGUCHI O, HASHIMOTO A, et al. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat Commun. 2015;6(1):7527. [37] MURAKAWA T, OKAMOTO K, OMIYA S, et al. A mammalian mitophagy receptor, Bcl2-L-13, recruits the ULK1 complex to induce mitophagy. Cell Rep. 2019;26(2): 338-345.e6. [38] MURAKAWA T, ITO J, RUSU MC, et al. AMPK regulates Bcl2-L-13-mediated mitophagy induction for cardioprotection. Cell Rep. 2024;43(12):115001. [39] GARCIA‐OROZCO A, MARTINEZ‐MAGAÑA I A, RIERA‐LEAL A, et al. Macrophage inhibitory factor (MIF) gene polymorphisms are associated with disease susceptibility and with circulating MIF levels in active non‐segmental vitiligo in patients from western Mexico. Mol Genet Genomic Med. 2020;8(10):e1416. [40] WANG Y, HU Y, WANG H, et al. Deficiency of MIF accentuates overloaded compression‐induced nucleus pulposus cell oxidative damage via depressing mitophagy. Oxid Med Cell Longev. 2021;2021(1):6192498. [41] MIYAZAWA M, TSUJI Y. Evidence for a novel antioxidant function and isoform-specific regulation of the human p66Shc gene. Mol Biol Cell. 2014;25(13):2116-2127. [42] 王涵,于志锋.力学刺激在破骨细胞分化中的作用[J].医用生物力学,2024,39(4): 775-782. [43] 莫亮,卫杨文祥,周月惠,等.剑叶龙血素A对破骨细胞分化影响的研究[J].中国骨质疏松杂志,2024,30(10):1405-1411. [44] 朱汉民,王松,肖文琳,等.线粒体自噬调控骨代谢[J].中国组织工程研究,2025, 29(8):1676-1683. [45] ZHOU H, CHEN P, ZHAO C, et al. Fraxin inhibits ovariectomized-induced bone loss and osteoclastogenesis by suppressing ROS activity. Int Immunopharmacol. 2025; 147:113871. [46] 宋世雷.淫羊藿苷通过Ca2+-CaM/CaMKⅡ信号介导LAP及典型自噬对酒精干预下破骨细胞的影响机制研究[D].南宁:广西中医药大学,2024. [47] 刘庆羊.PINK1/Parkin通路在骨保护素调控破骨细胞线粒体自噬中的作用机制[D].扬州:扬州大学,2019. [48] ZHANG K, JIN D, ZHAO X, et al. HIF-1α-induced mitophagy regulates the regenerative outcomes of stem cells in fat transplantation. Cell Transplant. 2023;32: 09636897231210750. [49] HONG Z, WANG H, ZHANG T, et al. The HIF-1/BNIP3 pathway mediates mitophagy to inhibit the pyroptosis of fibroblast-like synoviocytes in rheumatoid arthritis. Int Immunopharmacol. 2024;127:111378. [50] JANG JS, HONG SJ, MO S, et al. PINK1 restrains periodontitis-induced bone loss by preventing osteoclast mitophagy impairment. Redox Biol. 2024;69:103023. [51] 彭力,辜诗淇,梁木春,等.Sirt3在BMSCs成骨成脂双向命运分化调控的作用[J].四川大学学报(自然科学版),2024, 61(6):205-214. [52] 陈亚林,秦东旭.京尼平苷介导Sirt3分子改善大鼠蛛网膜下腔出血神经损伤[J].热带医学杂志,2024,24(9):1235-1240, 1365. [53] 盛东,殷震宇.基于SIRT3介导成骨细胞凋亡通路探讨茶多酚对骨质疏松疾病的干预研究[J].现代药物与临床, 2022, 37(7):1445-1451. [54] RICHARDSON KK, ADAM GO, LING W, et al. Mitochondrial protein deacetylation by SIRT3 in osteoclasts promotes bone resorption with aging in female mice. Mol Metab. 2024;88:102012. [55] GUO Y, JIA X, CUI Y, et al. Sirt3-mediated mitophagy regulates AGEs-induced BMSCs senescence and senile osteoporosis. Redox Biol. 2021;41:101915. [56] ZHU J, TANG Y, WU Q, et al. Mechanism of participation of osteocytes in the formation of osteoclasts under hypoxia. Hua Xi Kou Qiang Yi Xue Za Zhi. 2019; 37(5):463-468. [57] LEE SY, KIM SJ, PARK KH, et al. Differential but complementary roles of HIF-1α and HIF-2α in the regulation of bone homeostasis. Commun Biol. 2024;7(1):892. [58] CHENG M, LIU L, LAO Y, et al. MicroRNA-181a suppresses parkin-mediated mitophagy and sensitizes neuroblastoma cells to mitochondrial uncoupler-induced apoptosis. Oncotarget. 2016; 7(27):42274. [59] 高敬, 邵秉一. miR-181a调控骨髓间充质干细胞中OPG水平及对破骨细胞活性的影响[J].中国细胞生物学学报, 2017,39(1):44-51. [60] INDRIERI A, CARRELLA S, ROMANO A, et al. miR‐181a/b downregulation exerts a protective action on mitochondrial disease models . EMBO molecular medicine, 2019,11(5): e8734.doi:10.15252/emmm. 201708734 [61] 祝震亚,童蕾,陆燕群.miR-181a调控PINK1/Parkin通路对骨质疏松大鼠破骨细胞线粒体自噬的影响[J].解放军医学杂志,2022,47(6):569-578. [62] TAKEGAHARA N, KIM H, MIZUNO H, et al. Involvement of receptor activator of nuclear factor-κB ligand (RANKL)-induced incomplete cytokinesis in the polyploidization of osteoclasts. J Biol Chem. 2016;291(7):3439-3454. [63] XIANG Q, LI L, JI W, et al. Beyond resorption: osteoclasts as drivers of bone formation. Cell Regen. 2024;13(1):22. [64] HUANG T, WANG Y, YU Z, et al. Effect of mitophagy in the formation of osteomorphs derived from osteoclasts. Iscience. 2023; 26(5):106682. [65] YE F, WU A. The protective mechanism of SIRT1 in the regulation of mitochondrial biogenesis and mitochondrial autophagy in Alzheimer’s disease. J Alzheimers Dis. 2021;82(1):149-157. [66] SUN X, HAN Y, DONG C, et al. Daming capsule protects against myocardial infarction by promoting mitophagy via the SIRT1/AMPK signaling pathway. Biomed Pharmacother. 2022;151:113162. [67] ZHANG T, WANG L, DUAN X, et al. Sirtuins mediate mitochondrial quality control mechanisms: a novel therapeutic target for osteoporosis. Front Endocrinol. 2024; 14:1281213. [68] BONONI G, CITI V, LAPILLO M, et al. Sirtuin 1-activating compounds: discovery of a class of thiazole-based derivatives. Molecules. 2022;27(19):6535. [69] ISHIYAMA A, SUDA K, RAO X, et al. Angiopoietin-1 attenuates lipopolysaccharide-induced endotoxemia in a Hirschsprung’s disease murine model by improving intestinal vascular integrity: implications for treating postoperative Hirschsprung-associated enterocolitis. Pediatr Surg Int. 2024;40(1):277. [70] HOU J, HUANG X, SHANG L, et al. Reduced angiopoietin factor 2 levels are correlated with better cardiac function and prognosis in valvular heart disease. Braz J Cardiovasc Surg. 2022;38:104-149. [71] LANGHNOJA J, WEI X, AROUNLEUT P, et al. BIOL-17. Dissecting the expression and function of angiopoietin-1 in pediatric brain tumors. Neuro-Oncol. 2023;25(Suppl 1):i9. [72] JEONG BC, KIM HJ, BAE IH, et al. COMP-Ang1, a chimeric form of Angiopoietin 1, enhances BMP2-induced osteoblast differentiation and bone formation. Bone. 2010;46(2):479-486. [73] WANG R, LI H, XIE Z, et al. Development of a recombinant Ang1 variant with enhanced Tie2 binding and its application to attenuate sepsis in mice. Sci Adv. 2025;11(3):eads1796. [74] YAO N, MA Q, YI W, et al. Ang-1 promotes tumorigenesis and mediates the anti-cancer effects of Artesunate on Choroidal melanoma via the regulation of Akt/mTOR signaling pathway. Cytokine. 2024;184: 156771. [75] FRASCA L, SARUBBI A, LONGO F, et al. Remifentanil-Propofol-Ketamine-Based Total Intravenous Anesthesia with Spontaneous Breathing for Adult Rigid Bronchoscopy. J Clin Med. 2025;14(2):377. [76] LAZAROU M, SLITER DA, KANE LA, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524(7565):309-314. [77] YE P, PAN G, LI Y, et al. The role of remifentanil in regulating mitochondrial autophagy in osteoclasts was investigated based on PINK1/Parkin pathway. Cell Mol Biol. 2024;70(7):186-192. [78] LI J, FU SF, YANG Y, et al. Clinical practice of traditional Chinese medicine for the treatment of postmenopausal osteoporosis: a literature review. Climacteric. 2022;25(6): 562-569. [79] XU W, JIANG Y, WANG N, et al. Traditional Chinese Medicine as a promising strategy for the treatment of Alzheimer’s disease complicated with osteoporosis. Front Pharmacol. 2022;13:842101. [80] JOHN AA, XIE J, YANG YS, et al. AAV-mediated delivery of osteoblast/osteoclast-regulating miRNAs for osteoporosis therapy. Mol Ther Nucleic Acids. 2022;29:296-311. [81] AOKI S, SHIMIZU K, ITO K. Autophagy-dependent mitochondrial function regulates osteoclast differentiation and maturation. Biochem Biophys Res Commun. 2020;527(4):874-880. [82] SHEN J, GAO Y, DENG Y, et al. Eucommia ulmoides extract regulates oxidative stress to maintain calcium homeostasis and improve diabetic osteoporosis. Food Sci Nutr. 2024;12(10):8067-8083. [83] SHAO Y, CHEN S, ZHOU K, et al. Network pharmacology explores the mechanisms of Eucommia ulmoides cortex against postmenopausal osteoporosis. Medicine. 2022;101(19):e29257. [84] KIM HH, PARK SY, KIM KB, et al. A Study on the Effects of Eucommiae Cortex on Male Osteoporosis. Indian J Public Health. 2019;10(11). doi: 10.5958/0976-5506.2019.04286.4 [85] 杨波.人工虎骨粉通过AMPK对PINK 1/Parkin介导的线粒体自噬对膝骨关节炎软骨退变的研究 [D]. 兰州:甘肃中医药大学,2024. [86] GUPTA S, CASSEL SL, SUTTERWALA FS, et al. Regulation of the NLRP3 inflammasome by autophagy and mitophagy. Immunol Rev. 2025;329(1):e13410. [87] WU KKL, CHENG KY. A new role of the early endosome in restricting NLRP3 inflammasome via mitophagy. Autophagy. 2022;18(6):1475-1477. [88] YANG J, ZHAO M, ZENG T, et al. Shenmai injection improves lipid metabolism in post-myocardial infarction heart failure based on network pharmacology and experimental validation. Heliyon. 2024;10(21):e38648. [89] XU HH, JIANG ZH, HUANG CS, et al. Global metabolomic and lipidomic analysis reveals the potential mechanisms of hemolysis effect of Ophiopogonin D and Ophiopogonin D’in vivo. Chin Med. 2021;16:1-13. [90] LU Z, WU C, ZHU M, et al. Ophiopogonin D’induces RIPK1‑dependent necroptosis in androgen‑dependent LNCaP prostate cancer cells. Int J Oncol. 2020;56(2):439-447. [91] LEI S, FENG Y, HUANG P, et al. Ophiopogonin D′-induced mitophagy and mitochondrial damage are associated with dysregulation of the PINK1/Parkin signaling pathway in AC16 cells. Toxicology. 2022;477:153275. [92] WANG R, GAO C, YU M, et al. Mechanistic prediction and validation of Brevilin A Therapeutic effects in Lung Cancer. BMC Complement Med Ther. 2024;24(1):214. [93] ZHANG X, XIA Y, YANG L, et al. Brevilin A, a sesquiterpene lactone, inhibits the replication of influenza a virus in vitro and in vivo . Viruses. 2019;11(9):835. [94] LIU R, QU Z, LIN Y, et al. Brevilin A induces cell cycle arrest and apoptosis in nasopharyngeal carcinoma. Front Pharmacol. 2019;10:594. [95] QUE X, FAN J, CHEN D, et al. Brevilin A Inhibits Prostate Cancer Progression by Decreasing PAX5-Activated SOX4. Mol Biotechnol. 2025;67(5):2060-2071. [96] ZHOU R, WANG Y, LIU S, et al. Brevilin A, a novel BNIP3 inhibitor suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss via impairing mitophagy and mitochondrial metabolism. Phytomedicine. 2025;143:156774. [97] WOJDASIEWICZ P, BRODACKI S, CIEŚLICKA E, et al. Salidroside: A Promising Agent in Bone Metabolism Modulation. Nutrients. 2024;16(15):2387. [98] MENDJARGAL A, NARMANDAKH S, ZINAMYADAR M, et al. The inhibitory effect of salidroside on RANKL-induced osteoclast formation via NFκB suppression. In Vitro Cell Dev Biol Anim. 2025;61(1):59-66. [99] JIN Y, WANG Y, WANG C, et al. Salidroside inhibits osteoclast differentiation based on osteoblast-osteoclast interaction via HIF-1a pathway. Chin J Nat Med. 2025;23(5):572-584. [100] YAO H, XIANG L, HUANG Y, et al. Guizhi Shaoyao Zhimu granules attenuate bone destruction in mice with collagen-induced arthritis by promoting mitophagy of osteoclast precursors to inhibit osteoclastogenesis. Phytomedicine. 2023;118:154967. [101] HUANG Y, YAO H, TJAHJONO AW, et al. Si-Zhi Wan regulates osteoclast autophagy in osteoporosis through the AMPK signaling pathway to attenuate osteoclastogenesis. J Pharm Pharmacol. 2024;76(3):236-244. |
| [1] | Liu Wenlong, Dong Lei, Xiao Zhengzheng, Nie Yu. Finite element analysis of tibial prosthesis loosening after fixed-bearing unicompartmental knee arthroplasty for osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2191-2198. |
| [2] | Chen Long, Wang Xiaozhen, Xi Jintao, Lu Qilin. Biomechanical performance of short-segment screw fixation combined with expandable polyetheretherketone vertebral body replacement in osteoporotic vertebrae [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2226-2235. |
| [3] | Chen Huiting, Zeng Weiquan, Zhou Jianhong, Wang Jie, Zhuang Congying, Chen Peiyou, Liang Zeqian, Deng Weiming. Tail anchoring technique of vertebroplasty in treatment of osteoporotic vertebral compression fractures with intravertebral cleft: a finite element analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2145-2152. |
| [4] | Zeng Xuan, Weng Rui, Ye Shicheng, Tang Jiadong, Mo Ling, Li Wenchao. Two lumbar rotary manipulation techniques in treating lumbar disc herniation: a finite element analysis of biomechanical differences [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2153-2161. |
| [5] | Cheng Qisheng, Julaiti·Maitirouzi, Xiao Yang, Zhang Chenwei, Paerhati·Rexiti. Finite element analysis of novel variable-diameter screws in modified cortical bone trajectory of lumbar vertebrae [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2162-2171. |
| [6] | Hu Xiongke, Liu Shaohua, Tan Qian, Liu Kun, Zhu Guanghui. Shikonin intervention with bone marrow mesenchymal stem cells improves microstructure of femur in aged mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1609-1615. |
| [7] | Wu Zhilin, , He Qin, Wang Pingxi, Shi Xian, Yuan Song, Zhang Jun, Wang Hao . DYRK2: a novel therapeutic target for rheumatoid arthritis combined with osteoporosis based on East Asian and European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1569-1579. |
| [8] | Zhang Haiwen, Zhang Xian, Xu Taichuan, Li Chao. Bibliometric and visual analysis of the research status and trends of senescence in osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1580-1591. |
| [9] | Wen Guangwei, Zhen Yinghao, Zheng Taikeng, Zhou Shuyi, Mo Guoye, Zhou Tengpeng, Li Haishan, Lai Yiyi. Effects and mechanisms of isoginkgetin on osteoclastogenesis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1348-1358. |
| [10] | Cao Xinyan, Yu Zifu, Leng Xiaoxuan, Gao Shiai, Chen Jinhui, Liu Xihua. Effect of repetitive transcranial magnetic stimulation and transcranial direct current stimulation on motor function and gait in children with cerebral palsy: a network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1539-1548. |
| [11] | Guo Ying, Tian Feng, Wang Chunfang. Potential drug targets for the treatment of rheumatoid arthritis: large sample analysis from European databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1549-1557. |
| [12] | Huang Jie, Zeng Hao, Wang Wenchi, Lyu Zhucheng, Cui Wei. Visualization analysis of literature on the effect of lipid metabolism on osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1558-1568. |
| [13] | Yang Zhijie, Zhao Rui, Yang Haolin, Li Xiaoyun, Li Yangbo, Huang Jiachun, Lin Yanping, Wan Lei, HuangHongxing. Postmenopausal osteoporosis: predictive values of muscle mass, grip strength, and appendicular skeletal muscle index [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1073-1080. |
| [14] | Zhou Jian, Zhang Tao, Zhou Weili, Zhao Xingcheng, Wang Jun, Shen Jie, Qian Li, Lu Ming. Effects of resistance training on quadriceps mass and knee joint function in patients with osteoporosis and sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1081-1088. |
| [15] | Cao Wenqi, Feng Xiuzhi, Zhao Yi, Wang Zhimin, Chen Yiran, Yang Xiao, Ren Yanling. Effect of macrophage polarization on osteogenesis-angiogenesis coupling in type 2 diabetic osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 917-925. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||