Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (16): 4240-4252.doi: 10.12307/2026.734
Previous Articles Next Articles
Potential effects of cuproptosis-related genes in the onset of ankylosing spondylitis: a multi-omics Mendelian randomization study
Song Zhichao, Qi Wenrong, Meng Peng, Zhang Yanyan
- Yantai Hospital of Traditional Chinese Medicine, Yantai 264000, Shandong Province, China
-
Received:2025-07-09Accepted:2025-08-30Online:2026-06-08Published:2025-11-29 -
Contact:Zhang Yanyan, MS, Attending physician, Yantai Hospital of Traditional Chinese Medicine, Yantai 264000, Shandong Province, China -
About author:Song Zhichao, MS, Yantai Hospital of Traditional Chinese Medicine, Yantai 264000, Shandong Province, China -
Supported by:Binzhou Medical University Teaching Reform Project, No. SJJY201914 (to MP); Yantai Science and Technology Plan Project, No. 2020MSGY087 (to SZC [project participant])
CLC Number:
Cite this article
Song Zhichao, Qi Wenrong, Meng Peng, Zhang Yanyan. Potential effects of cuproptosis-related genes in the onset of ankylosing spondylitis: a multi-omics Mendelian randomization study[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4240-4252.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
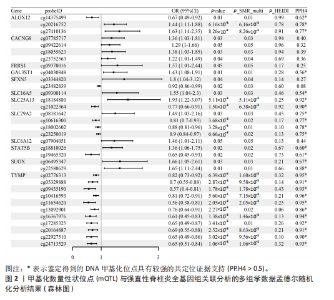
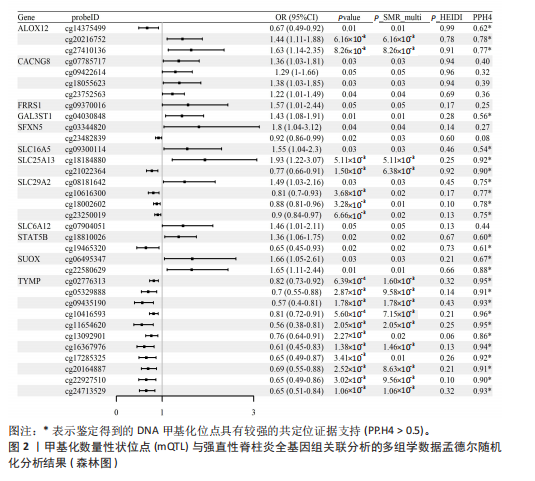
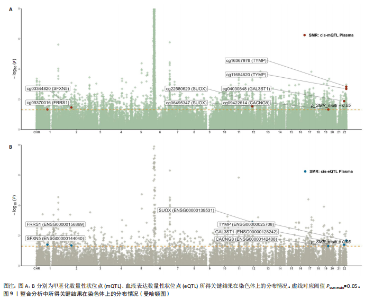
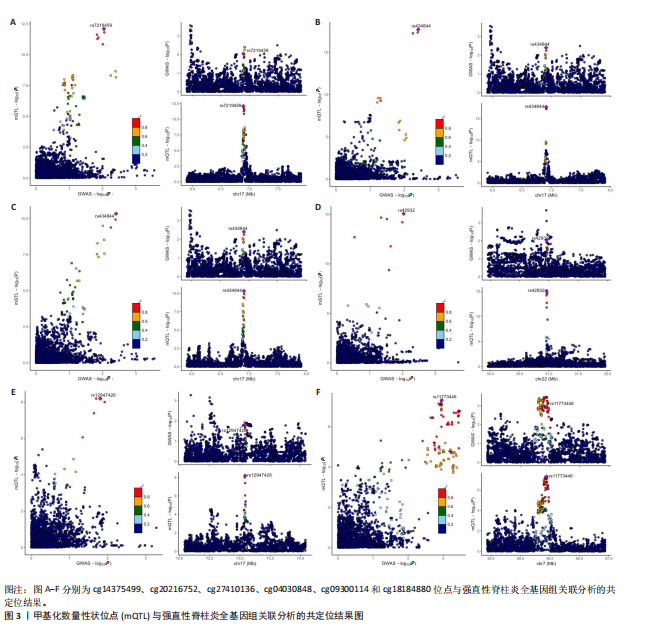
2.1 整合强直性脊柱炎全基因组关联分析和铜死亡相关的血液mQTL数据 从分析数据中鉴定了335个与强直性脊柱炎相关的DNA甲基化位点,对应191个基因(PSMR < 0.05,PSMR multi < 0.05,PHEIDI > 0.05)。鉴于上述位点数量较多,展示较为困难,该部分结果结合血液eQTL与强直性脊柱炎全基因组关联分析间的多组学数据孟德尔随机化分析结果,仅选择所在基因的表达与强直性脊柱炎风险亦显著相关的关键DNA甲基化位点进行了展示,DNA甲基化位点与强直性脊柱炎间的因果关系,见表1和图2。如位于花生四烯酸-12-脂加氧酶(ALOX12)的DNA甲基化位点cg14375499,其甲基化水平与强直性脊柱炎风险呈负相关[OR=0.67,95%CI(0.49-0.92)]。此外,位于同一基因的不同DNA甲基化位点,效应评估方向可能是不一致的,如另一位于花生四烯酸-12-脂加氧酶的DNA甲基化位点cg20216752与强直性脊柱炎风险呈正相关[OR= 1.44,95%CI( 1.11-1.88)]。共定位分析结果显示,上述鉴定到的特征信号中有200个位点(对应105个基因)在位点对应单核苷酸多态性的共定位区域窗口中具有较强的共定位证据支持(PP.H4 > 0.5)。鉴于位点数量较多,展示较为困难,该部分共定位结果仅展示图2中PP.H4 > 0.5的前6个位点,"
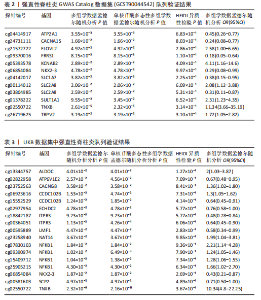
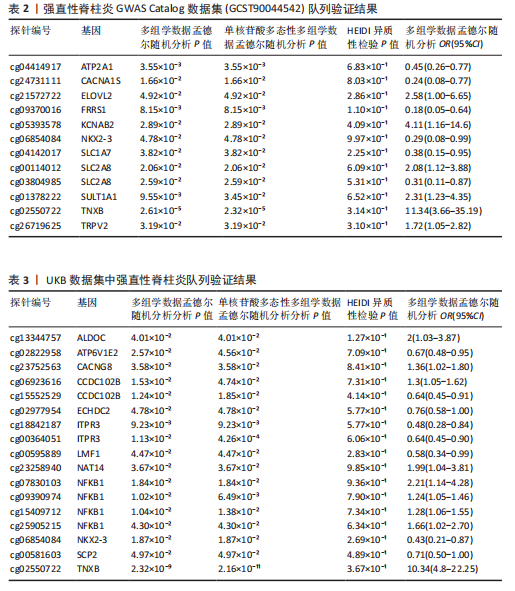
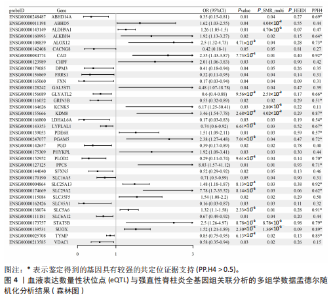
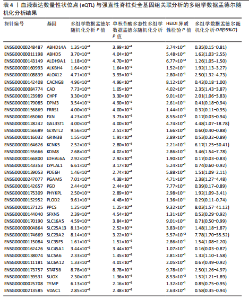
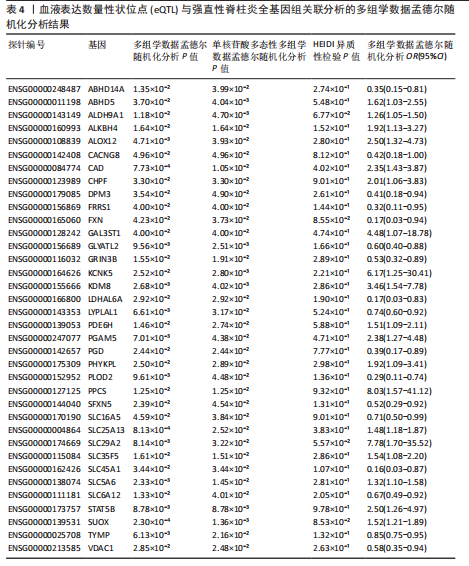
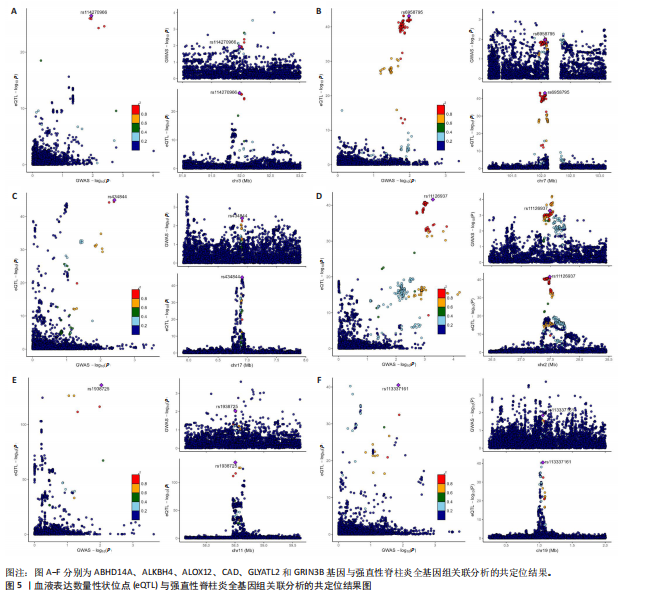
见图3。上述结果中肌质/内质网钙ATP酶(ATP2A1)(cg04414917)、钙通道电压依赖L型α1S亚基(CACNA1S)(cg24731111)等12个甲基化位点(对应11个基因)在GWAS Catalog (GCST90044542)强直性脊柱炎队列中得到验证(PSMR < 0.05,PSMR multi < 0.05, PHEIDI > 0.05),见表2。果糖二磷酸醛缩酶C(ALDOC)(cg13344757)、V型质子泵V1区E2亚基(ATP6V1E2)(cg02822958)等17个甲基化位点(对应12个基因)在UKB-强直性脊柱炎队列中得到验证(PSMR < 0.05 ,PSMR multi < 0.05,PHEIDI > 0.05),见表3。 2.2 整合强直性脊柱炎全基因组关联分析和铜死亡相关的血液eQTL数据 鉴定出36个铜死亡相关基因与强直性脊柱炎风险相关(PSMR < 0.05,PSMR multi < 0.05,PHEIDI > 0.05),见表4和图4。其中,19个基因的表达水平与强直性脊柱炎风险呈负相关。共定位分析结果显示,上述鉴定到的特征信号中19个基因在共定位区域窗口中具有较强的证据支持(PP.H4 > 0.5),见图4。eQTL与强直性脊柱炎关联的单核苷酸多态性共定位结果图见图5,鉴于基因数量较多,展示较为困难,该部分共定位结果仅展示图4中PP.H4 > 0.5的前6个位点。验证阶段发现,铁螯合还原酶1(FRRS1)(PSMR=4.87×10-3,PSMR multi=4.87×10-2,PHEIDI=0.12)和软骨蛋白聚合因子(CHPF)(PSMR=4.11×10-2,PSMR multi=4.11×10-2,PHEIDI=0.96)基因在GCST90044542队列中得到验证,而在UKB数据集队列中以上探索结果则未得到验证。"
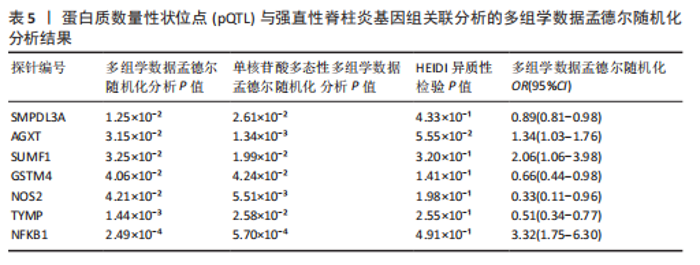
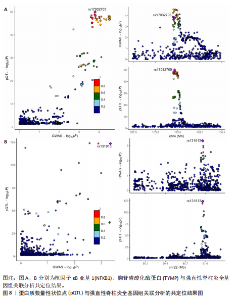
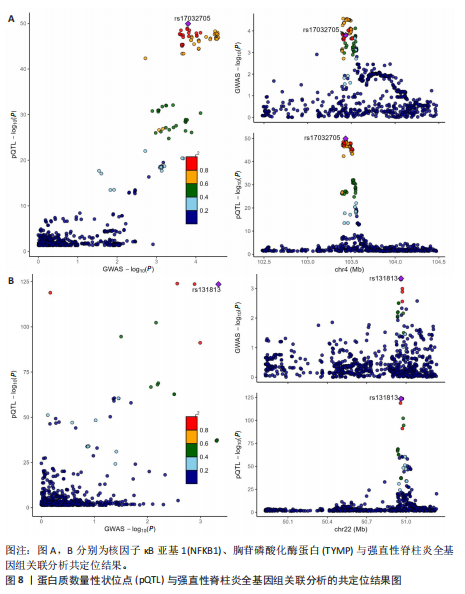
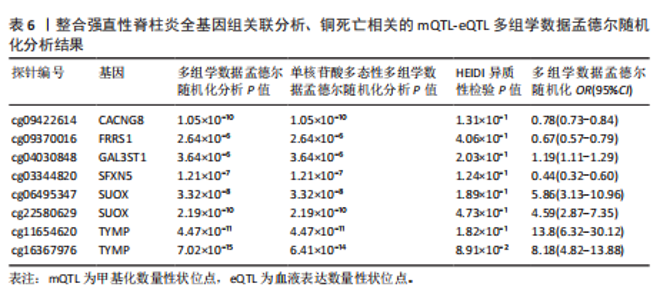
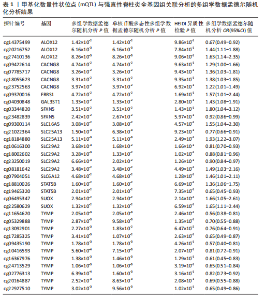
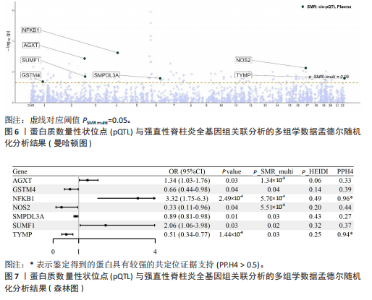
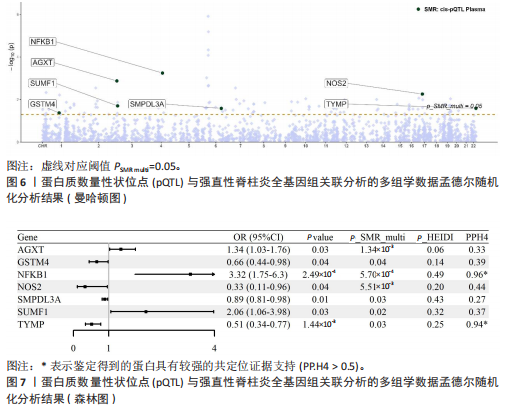
2.3 整合强直性脊柱炎全基因组关联分析和铜死亡相关的血液pQTL数据 铜死亡相关蛋白丰度与强直性脊柱炎的因果关系见表5,共鉴定出7个铜死亡相关蛋白与强直性脊柱炎风险相关(PSMR < 0.05,PSMR multi < 0.05,PHEIDI > 0.05)。这7个蛋白在染色体上的分布情况见图6。除丙氨酸乙醛酸转氨酶(AGXT)、硫酸酯酶修饰因子1 (SUMF1)、核因子kB亚基1(NFKB1)外,其余蛋白丰度与强直性脊柱炎风险均呈负相关,见图7。共定位分析结果显示,上述鉴定到的特征信号中核因子kB亚基1和胸苷磷酸化酶(TYMP)在共定位区域窗口中具有较强的共定位证据支持(PP.H4 > 0.5),共定位结果图见图8。验证结果显示,核因子kB亚基1(PSMR=6.67×10-3,PSMR multi=3.13×10-2,PHEIDI=0.63)、一氧化氮合酶2(NOS2)(PSMR=8.75×10-3,PSMR multi=3.24×10-2,PHEIDI=0.25)共2个编码蛋白在UKB队列中得到验证,但没有蛋白在GCST90044542队列中得到验证。 2.4 整合强直性脊柱炎全基因组关联分析、铜死亡相关的血液mQTL和eQTL数据 基于铜死亡相关血液eQTL、mQTL与强直性脊柱炎全基因组关联分析的多组学数据孟德尔随机化分析中所得关键结果,作者认为铁螯合还原酶1、侧链蛋白5、SLC25A13、SLC29A2、SLC6A12、亚硫酸氧化酶、花生四烯酸-12-脂加氧酶、信号转导和转录激活因子(STAT5B)、SLC16A5、癌胚抗原相关细胞附着分子8、半乳糖-3-O-磺基转移酶1、胸苷磷酸化酶基因可能与强直性脊柱炎有因果关联。进一步以mQTL作为暴露条件,eQTL作为结局的情况下进行多组学数据孟德尔随机化分析,以探索上述交叉结果中DNA甲基化位点甲基化对所在基因表达是否具有显著的调控作用。结果发现癌胚抗原相关细胞附着分子8(cg09422614)、铁螯合还原酶1(cg09370016)、半乳糖-3-O-磺基转移酶1(cg04030848)、侧链蛋白5(cg03344820)、亚硫酸氧化酶(cg06495347、cg22580629)、胸苷磷酸化酶(cg11654620、cg16367976)在"

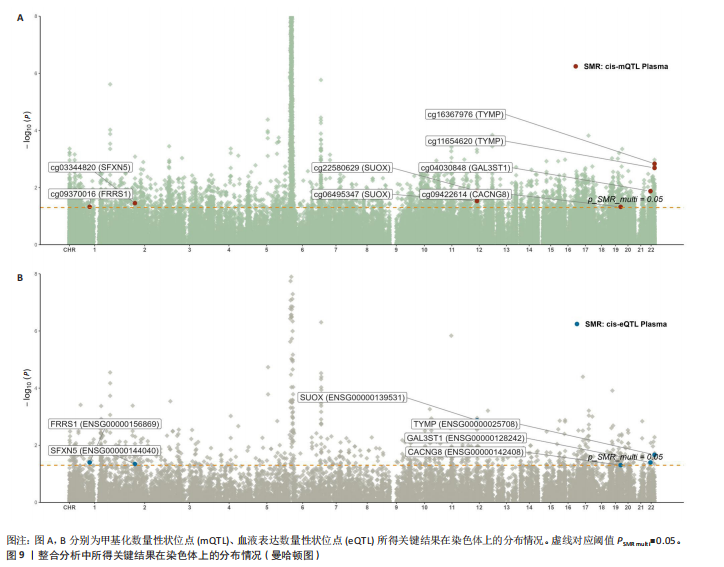
上述结果中均得到了显著验证(PSMR < 0.05,PSMR multi < 0.05,PHEIDI > 0.05),见表6。 2.5 整合强直性脊柱炎全基因组关联分析、铜死亡相关的血液eQTL和pQTL数据 综合考虑铜死亡相关血液pQTL与强直性脊柱炎全基因组关联分析的多组学数据孟德尔随机化分析结果,作者认为胸苷磷酸化酶基因可能与强直性脊柱炎有因果关联。进一步的在血液eQTL为暴露条件,pQTL为结局的情况下进行多组学数据孟德尔随机化分析,以探索上述交叉结果中基因对所在蛋白丰度是否具有显著的调控作用。结果显示多组学数据孟德尔随机化基因未得到显著验证。 2.6 整合多组学水平证据 基于以上分析结果,作者认为癌胚抗原相关细胞附着分子8(cg09422614)、铁螯合还原酶1(cg09370016)、半乳糖-3-O-磺基转移酶1(GAL3ST1,cg04030848)、侧链蛋白5(cg03344820)、亚硫酸氧化酶(cg06495347、cg22580629)、胸苷磷酸化酶(cg11654620、cg16367976)可能与强直性脊柱炎有因果关联,所述关键结果位点、基因在染色体上的分布情况见图9。上述基因在血液mQTL、eQTL的多组学数据孟德尔随机化分析中均验证显著。半乳糖-3-O-磺基转移酶1的甲基化位点cg04030848在mQTL与强直性脊柱炎全基因组关联分析的共定位分析中得到了较强共定位证据支持(PP.H4 > 0.5),亚硫酸氧化酶和胸苷磷酸化酶则在eQTL与强直性脊柱炎全基因组关联分析的共定位分析中得到了较强共定位证据支持(PP.H4 > 0.5)。上述基因及位点在mQTL-eQTL分析中均得到显著性验证。通过OR值对风险相关性及调控方向进行判断,结果发现cg09422614、cg09370016和cg03344820位点的甲基化水平与强直性脊柱炎风险呈正相关,癌胚抗原相关细胞附着分子8、铁螯合还原酶1和侧链蛋白5基因的表达水平与强直性脊柱炎风险呈负相关,因此,cg09422614、cg09370016和cg03344820位点甲基化分别负向调控各自对应的基因表达水平。而cg04030848、cg06495347和cg22580629位点的甲基化水平与强直性脊柱炎风险呈正相关,半乳糖-3-O-磺基转移酶1和亚硫酸氧化酶基因的表达水平与强直性脊柱炎风险呈正相关,因此,cg04030848、cg06495347和cg22580629位点甲基化分别正向调控所在基因的表达水平。此外,还发现cg11654620和cg16367976位点的甲基化水平与强直性脊柱炎风险呈负相关,而胸苷磷酸化酶基因的表达水平则与强直性脊柱炎风险呈负相关,因此,cg11654620和cg16367976位点甲基化正向调控胸苷磷酸化酶基因的表达水平。综上,可能存在假定的机制:cg09422614、cg09370016和cg03344820位点较高的甲基化水平分别抑制了癌胚抗原相关细胞附着分子8、铁螯合还原酶1和侧链蛋白5基"
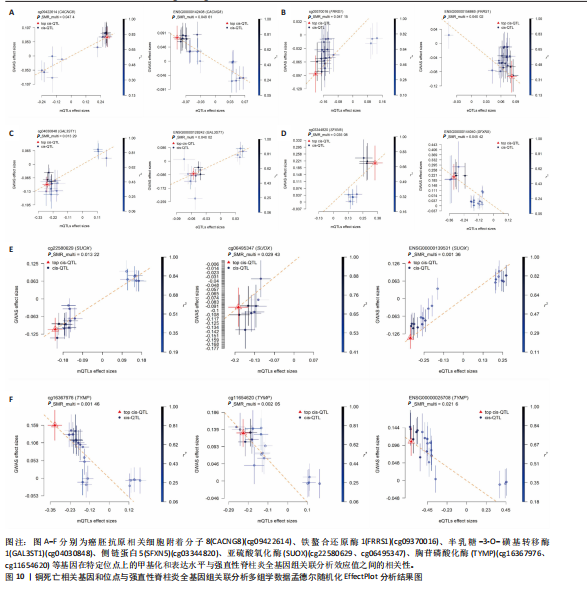
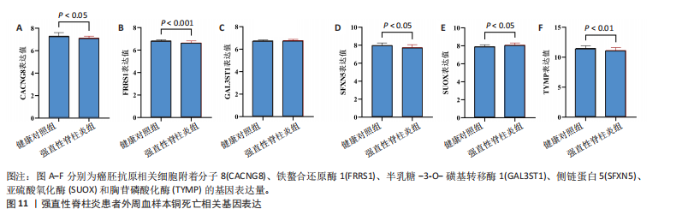
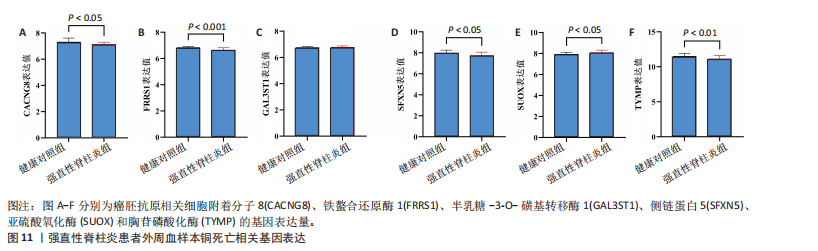
因的表达水平,从而升高了强直性脊柱炎风险;cg04030848、cg06495347和cg22580629位点较高的甲基化水平分别促进了半乳糖-3-O-磺基转移酶1和亚硫酸氧化酶基因的表达水平,从而升高了强直性脊柱炎风险;cg11654620和cg16367976位点较低的甲基化水平抑制了胸苷磷酸化酶基因的表达水平,从而升高了强直性脊柱炎风险。 通过多组学数据孟德尔随机化 EffectPlot分析展示了癌胚抗原相关细胞附着分子8(cg09422614)、铁螯合还原酶1(cg09370016)、半乳糖-3-O-磺基转移酶1(cg04030848)、侧链蛋白5(cg03344820)、亚硫酸氧化酶(cg06495347、cg22580629)和胸苷磷酸化酶(cg11654620、cg16367976)在特定位点上的甲基化或表达水平与全基因组关联分析效应值之间存在较强的相关性,显示了甲基化、基因表达与疾病风险之间的潜在因果关系(PSMR multi < 0.05),见图10。这些结果进一步支持了甲基化和基因表达在强直性脊柱炎相关遗传风险中的作用。 2.7 强直性脊柱炎患者外周血样本铜死亡相关基因表达情况 获取16例健康人群和16例强直性脊柱炎患者的外周血样本测序数据集GSE25101,探索已鉴定到的铜死亡相关基因癌胚抗原相关细胞附着分子8、铁螯合还原酶1、半乳糖-3-O-磺基转移酶1、侧链蛋白5、亚硫酸氧化酶和胸苷磷酸化酶的表达情况。结果显示,与健康对照组相比,强直性脊柱炎组癌胚抗原相关细胞附着分子8、铁螯合还原酶1、侧链蛋白5和胸苷磷酸化酶的基因表达均降低(P < 0.05),亚硫酸氧化酶的基因表达升高(P < 0.05),见图11。上述结果表明,这些已鉴定的铜死亡相关基因在强直性脊柱炎发病机制中具有一定的调控作用,可能参与强直性脊柱炎的病理进程。"
| [1] TSVETKOV P, COY S, PETROVA B, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375(6586):1254-1261. [2] BROWN MA, KENNA T, WORDSWORTH BP. Genetics of ankylosing spondylitis--insights into pathogenesis. Nat Rev Rheumatol. 2016;12(2):81-91. [3] SOLIER S, MÜLLER S, CAÑEQUE T, et al. A druggable copper-signalling pathway that drives inflammation. Nature. 2023; 617(7960):386-394. [4] AIGINGER P, KOLARZ G, WILLVONSEDER R. Copper in ankylosing spondylitis and rheumatoid arthritis. Scand J Rheumatol. 1978;7(2):75-78. [5] COBINE PA, BRADY DC. Cuproptosis: Cellular and molecular mechanisms underlying copper-induced cell death. Mol Cell. 2022; 82(10):1786-1787. [6] COBINE PA, MOORE SA, LEARY SC. Getting out what you put in: Copper in mitochondria and its impacts on human disease. Biochim Biophys Acta Mol Cell Res. 2021;1868(1):118867. [7] 朱赛雅,刘晶,余晨.线粒体铜稳态失衡与纤维化疾病的研究进展[J].生理学报, 2024,76(4):597-604. [8] ZHOU Y, ZHANG L. The interplay between copper metabolism and microbes: in perspective of host copper-dependent ATPases ATP7A/B. Front Cell Infect Microbiol. 2023;13:1267931. [9] ZHANG P, CHEN H, ZHANG Y, et al. Dry and wet experiments reveal diagnostic clustering and immune landscapes of cuproptosis patterns in patients with ankylosing spondylitis. Int Immunopharmacol. 2024; 127:111326. [10] KARDOS J, HÉJA L, SIMON Á, et al. Copper signalling: causes and consequences. Cell Commun Signal. 2018;16(1):71. [11] JAYSON MI, DAVIS P, WHICHER JT, et al. Serum copper and caeruloplasmin in ankylosing spondylitis, systemic sclerosis, and morphea. Ann Rheum Dis. 1975;35(5): 443-445. [12] FAN J, LIU Q, CHEN T, et al. Identification of cuproptosis-related genes related to the progression of ankylosing spondylitis by integrated bioinformatics analysis. Medicine (Baltimore). 2024;103(35):e38313. [13] WU Y, ZENG J, ZHANG F, et al. Integrative analysis of omics summary data reveals putative mechanisms underlying complex traits. Nat Commun. 2018;9(1):918. [14] VÕSA U, CLARINGBOULD A, WESTRA HJ, et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat Genet. 2021; 53(9):1300-1310. [15] SUN BB, CHIOU J, TRAYLOR M, et al. Plasma proteomic associations with genetics and health in the UK Biobank. Nature. 2023; 622(7982):329-338. [16] PAIRO-CASTINEIRA E, RAWLIK K, BRETHERICK AD, et al. GWAS and meta-analysis identifies 49 genetic variants underlying critical COVID-19. Nature. 2023; 617(7962):764-768. [17] ZHU Z, ZHANG F, HU H, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. 2016;48(5):481-487. [18] ORTEGA A, TARAZÓN E, ROSELLÓ-LLETÍ E, et al. Patients with Dilated Cardiomyopathy and Sustained Monomorphic Ventricular Tachycardia Show Up-Regulation of KCNN3 and KCNJ2 Genes and CACNG8-Linked Left Ventricular Dysfunction. PLoS One. 2015; 10(12):e0145518. [19] BAI WJ, LUO XG, JIN BH, et al. Deficiency of transmembrane AMPA receptor regulatory protein γ-8 leads to attention-deficit hyperactivity disorder-like behavior in mice. Zool Res. 2022;43(5):851-870. [20] WU X, GAO L, ZhOU K, et al. Deposition and transport of trace mineral elements were affected by stocking density in fattening pigs. J Trace Elem Med Biol. 2018;50:566-571. [21] WANG H, LI J, ZI X, et al. Comprehensive analysis of cuproptosis-related genes on bladder cancer prognosis, tumor microenvironment invasion, and drug sensitivity. Front Oncol. 2023;13:1116305. [22] LIU H, LIU L, LIU Q, et al. LncRNA HOXD-AS1 affects proliferation and apoptosis of cervical cancer cells by promoting FRRS1 expression via transcription factor ELF1. Cell Cycle. 2022;21(4):416-421. [23] GUERRA LN, SUAREZ C, SOTO D, et al. GAL3ST2 from mammary gland epithelial cells affects differentiation of 3T3-L1 preadipocytes. Clin Transl Oncol. 2015; 17(7):511-520. [24] ROBINSON CM, POON BPK, KANO Y, et al. A Hypoxia-Inducible HIF1-GAL3ST1-Sulfatide Axis Enhances ccRCC Immune Evasion via Increased Tumor Cell-Platelet Binding [published correction appears in Mol Cancer Res. 2021;19(4):739. [25] 翁勰,肖芦山,邹雪晶,等.GAL3ST1对肝细胞癌肿瘤进展的影响及机制研究[J].临床肿瘤学杂志,2022,27(6):488-494. [26] MENG Q, HU X, ZHAO X, et al. A circular network of coregulated sphingolipids dictates lung cancer growth and progression. EBioMedicine. 2021;66: 103301. [27] CHEN L, ELIZALDE M, DUBOIS LJ, et al. GAL3ST1 Deficiency Reduces Epithelial-Mesenchymal Transition and Tumorigenic Capacity in a Cholangiocarcinoma Cell Line. Int J Mol Sci. 2024;25(13):7279. [28] NAITO Y, AKIBA J, KINJO Y, et al. Predictive and Prognostic Value of SUOX Expression in Pancreatic Ductal Adenocarcinoma. Anticancer Res. 2022;42(8):4145-4151. [29] TOKISAWA S, KONDO R, NAKAYAMA M, et al. Clinicopathological significance of sulfite oxidase expression in surgically resected lung adenocarcinoma. Med Mol Morphol. 2025;58(2):106-113. [30] JACKSON TD, HOCK DH, FUJIHARA KM, et al. The TIM22 complex mediates the import of sideroflexins and is required for efficient mitochondrial one-carbon metabolism. Mol Biol Cell. 2021;32(6):475-491. [31] ZHANG H, MENG L, LIU Y, et al. Sfxn5 Regulation of Actin Polymerization for Neutrophil Spreading Depends on a Citrate-Cholesterol-PI(4,5)P2 Pathway. J Immunol. 2023;211(3):462-473. [32] JIN Q, REN F, SONG P. Innovate therapeutic targets for autoimmune diseases: insights from proteome-wide mendelian randomization and Bayesian colocalization. Autoimmunity. 2024;57(1):2330392. [33] TAO Y, HUA G, MIN S, et al. Verification of biological markers of subacute cutaneous lupus erythematosus via TMT labelling proteomics combined with transcriptome data. Ann Med. 2025;57(1):2500696. [34] PALADHI A, DARIPA S, MONDAL I, et al. Targeting thymidine phosphorylase alleviates resistance to dendritic cell immunotherapy in colorectal cancer and promotes antitumor immunity. Front Immunol. 2022;13:988071. [35] YOU W, LIN Y, LIU M, et al. Investigating potential novel therapeutic targets and biomarkers for ankylosing spondylitis using plasma protein screening. Front Immunol. 2024;15:1406041. |
| [1] | Guo Ying, Tian Feng, Wang Chunfang. Potential drug targets for the treatment of rheumatoid arthritis: large sample analysis from European databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1549-1557. |
| [2] | Wu Zhilin, , He Qin, Wang Pingxi, Shi Xian, Yuan Song, Zhang Jun, Wang Hao . DYRK2: a novel therapeutic target for rheumatoid arthritis combined with osteoporosis based on East Asian and European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1569-1579. |
| [3] | Liu Hongtao, Wu Xin, Jiang Xinyu, Sha Fei, An Qi, Li Gaobiao. Causal relationship between age-related macular degeneration and deep vein thrombosis: analysis based on genome-wide association study data [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1602-1608. |
| [4] | Gao Zengjie, , Pu Xiang, Li Lailai, Chai Yihui, Huang Hua, Qin Yu. Increased risk of osteoporotic pathological fractures associated with sterol esters: evidence from IEU-GWAS and FinnGen databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1302-1310. |
| [5] | Liu Fengzhi, Dong Yuna, Tian Wenyi, Wang Chunlei, Liang Xiaodong, Bao Lin. Gene-predicted associations between 731 immune cell phenotypes and rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1311-1319. |
| [6] | Zhang Cuicui, Chen Huanyu, Yu Qiao, Huang Yuxuan, Yao Gengzhen, Zou Xu. Relationship between plasma proteins and pulmonary arterial hypertension and potential therapeutic targets [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1331-1340. |
| [7] | Zeng Hao, Sun Pengcheng, Chai Yuan, Huang Yourong, Zhang Chi, Zhang Xiaoyun. Association between thyroid function and osteoporosis: genome-wide data analysis of European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1019-1027. |
| [8] | Rong Xiangbin, , Zheng Haibo, Mo Xueshen, Hou Kun, Zeng Ping, . Plasma metabolites, immune cells, and hip osteoarthritis: causal inference based on GWAS data from European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1028-1035. |
| [9] | He Qiwang, , , Chen Bo, Liang Fuchao, Kang Zewei, Zhou Yuan, Ji Anxu, Tang Xialin, . Relationship between Alzheimer’s disease and sarcopenia and body mass index: analysis of GWAS datasets for European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1036-1046. |
| [10] | Ding Yu, Chen Jingwen, Chen Xiuyan, Shi Huimin, Yang Yudie, Zhou Meiqi, Cui Shuai, . Circulating inflammatory proteins and myocardial hypertrophy: large sample analysis of European populations from GWAS Catalog and FinnGen databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1047-1057. |
| [11] | Zhao Feifan, Cao Yujing. An artificial neural network model of ankylosing spondylitis and psoriasis shared genes and machine learning-based mining and validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 770-784. |
| [12] | Liu Chu, Qiu Boyuan, Tong Siwen, He Linyuwei, Chen Haobo, Ou Zhixue. A genetic perspective reveals the relationship between blood metabolites and osteonecrosis: an analysis of information from the FinnGen database in Finland [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 785-794. |
| [13] | Li Wei, Chai Jinlian, Zhang Bochun, Li Guangzheng, Liu Xiaochen, Wei Chuanfu, Chen Ning, Luo Di, Li Gang, Liang Xuezhen. Preventive effect of lipid-lowering drug targets on the risk of osteonecrosis: genetic information analysis based on the FinnGen and GLGC databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4508-4516. |
| [14] | Li Ruiying, Xia Hong. Visual analysis of cuproptosis research: global landscape of hotspots and frontiers [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4529-4541. |
| [15] | Liu Enxu, Sun Yu, Duan Jiahao, Yang Lei, Jiang Haobo, Yang Shaofeng. Bidirectional causal interplay between Epstein-Barr virus and ankylosing spondylitis: data analysis based on the UK Biobank and FinnGen databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4542-4547. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||