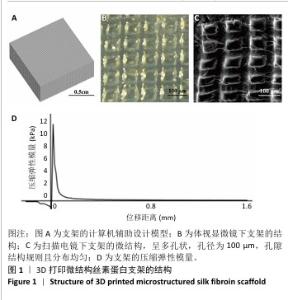
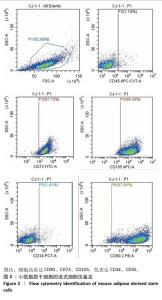
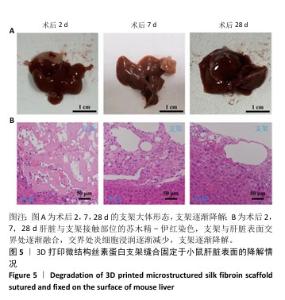
Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (14): 3618-3625.doi: 10.12307/2026.048
Previous Articles Next Articles
Preparation and characterization of 3D printed microstructured silk fibroin scaffold for liver injury repair
Shi Xiaonan1, 2, Wu Xuan1, Zhang Daxu2, Hu Jingjing1, 2, Zheng Yazhe2, Liu Yutong1, Zhao Shuo1, 2, Li Weilong1, 2, Ye Shujun1, Wang Jingyi1, Yan Li1
- 1Department of Geriatrics, Second Medical Center, Chinese PLA General Hospital, Beijing 100853, China; 2Institute of Orthopedics of First Medical Center, Beijing Key Lab of Regenerative Medicine in Orthopedics, Key Laboratory of Musculoskeletal Trauma & War Injuries PLA, Chinese PLA General Hospital, Beijing 100853, China
-
Received:2025-03-10Accepted:2025-04-19Online:2026-05-18Published:2025-09-11 -
Contact:Yan Li, MD, Chief physician, Professor, Department of Geriatrics, Second Medical Center, Chinese PLA General Hospital, Beijing 100853, China -
About author:Shi Xiaonan, Master candidate, Department of Geriatrics, Second Medical Center, Chinese PLA General Hospital, Beijing 100853, China; Institute of Orthopedics of First Medical Center, Beijing Key Lab of Regenerative Medicine in Orthopedics, Key Laboratory of Musculoskeletal Trauma & War Injuries PLA, Chinese PLA General Hospital, Beijing 100853, China -
Supported by:National Key Research and Development Plan Project, No. 2019YFA0110600 (to YL); National Natural Science Foundation of China, No. 31971263 (to YL)
CLC Number:
Cite this article
Shi Xiaonan, Wu Xuan, Zhang Daxu, Hu Jingjing, Zheng Yazhe, Liu Yutong, Zhao Shuo, Li Weilong, Ye Shujun, Wang Jingyi, Yan Li. Preparation and characterization of 3D printed microstructured silk fibroin scaffold for liver injury repair[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3618-3625.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
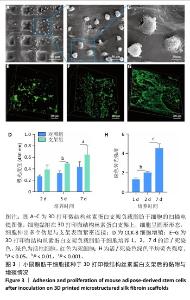
2.3 支架表面脂肪干细胞的黏附情况 扫描电镜下可见脂肪干细胞黏附在3D打印微结构丝素蛋白支架上,细胞大小为20-30 μm,细胞呈圆形形态,细胞伸出多个伪足与支架表面紧密连接,处于初期活跃阶段(图3A-C)。 2.4 支架的细胞毒性检测结果 CCK-8检测结果显示,随着培养时间的延长,对照组与支架组细胞数量逐渐增加,支架组培养5,7 d的细胞数量明显高于对照组(P < 0.05,P < 0.01),见图3D。表明3D打印微结构丝素蛋白支架没有细胞毒性,具有良好的生物相容性。 2.5 脂肪干细胞在支架上的增殖能力检测结果 活/死细胞染色结果显示,脂肪干细胞可以黏附在3D打印微结构丝素蛋白支架表面并进行增殖,在支架内分布逐渐均匀(图3E-H)。"

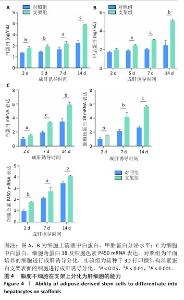
2.6 脂肪干细胞在支架上分化能力检测结果 成肝诱导培养2,5,7,14 d后,两组细胞上清中白蛋白及甲胎蛋白分泌量逐渐增加,并且实验组细胞上清中白蛋白及甲胎蛋白分泌量高于对照组(P < 0.05,P < 0.01,P < 0.001),见图4A,B。RT-PCR检测结果显示,成肝诱导培养2,7,14 d后,两组细胞中白蛋白、细胞角蛋白18、细胞色素 P450 mRNA表达逐渐升高,并且实验组细胞中白蛋白、细胞角蛋白18、细胞色素 P450 mRNA表达均高于对照组(P < 0.05,P < 0.01,P < 0.001),见图4C。表明3D打印微结构丝素蛋白支架可在成肝诱导培养环境中促进脂肪干细胞分化为肝细胞样细胞。 "

| [1] LI Y, LU L, CAI X. Liver Regeneration and Cell Transplantation for End-Stage Liver Disease. Biomolecules. 2021;11(12):1907. [2] LIU P, MAO Y, XIE Y, et al. Stem cells for treatment of liver fibrosis/cirrhosis: clinical progress and therapeutic potential. Stem Cell Res Ther. 2022;13(1):356. [3] COCCOLINI F, COIMBRA R, ORDONEZ C, et al. Liver trauma: WSES 2020 guidelines. World J Emerg Surg. 2020;15(1):24. [4] TIAN Y, ZHANG J, JIA Z, et al. Biomimetic mineralized mesenchymal stem cell-derived exosomes for dual modulation of ferroptosis and lactic acid-driven inflammation in acute liver injury therapy. J Colloid Interface Sci. 2025;687:489-506. [5] LUO J, LI J, LI P, et al. Acute-on-chronic liver failure: far to go-a review. Crit Care. 2023;27(1):259. [6] SARIN SK, CHOUDHURY A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13(3):131-149. [7] BROWN RS, FISHER RA, SUBRAMANIAN RM, et al. Artificial Liver Support Systems in Acute Liver Failure and Acute-on-Chronic Liver Failure: Systematic Review and Meta-Analysis. Crit Care Explor. 2025;7(1):e1199. [8] KJAERGARD LL, LIU J, ALS-NIELSEN B, et al. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. JAMA. 2003;289(2):217-222. [9] GEETHA BAI R, MUTHOOSAMY K, MANICKAM S, et al. Graphene-based 3D scaffolds in tissue engineering: fabrication, applications, and future scope in liver tissue engineering. Int J Nanomedicine. 2019;14:5753-5783. [10] JIN Y, ZHANG J, CHEN X, et al. 3D printing incorporating gold nanozymes with mesenchymal stem cell-derived hepatic spheroids for acute liver failure treatment. Biomaterials. 2025;315:122895. [11] ZHANG J, ZHAO X, LIANG L, et al. A decade of progress in liver regenerative medicine. Biomaterials. 2018;157:161-176. [12] WANG YH, CHEN EQ. Mesenchymal Stem Cell Therapy in Acute Liver Failure. Gut Liver. 2023;17(5):674-683. [13] FENG L, WANG Y, FU Y, et al. Stem Cell-Based Strategies: The Future Direction of Bioartificial Liver Development. Stem Cell Rev Rep. 2024; 20(3):601-616. [14] SHAH S, GHOSH D, OTSUKA T, et al. Classes of Stem Cells: From Biology to Engineering. Regen Eng Transl Med. 2024;10(3):309-322. [15] EOM Y W, YOON Y, BAIK SK. Mesenchymal stem cell therapy for liver disease: current status and future perspectives. Curr Opin Gastroenterol. 2021;37(3):216-223. [16] YAN Y, FANG J, WEN X, et al. Therapeutic applications of adipose-derived mesenchymal stem cells on acute liver injury in canines. Res Vet Sci. 2019;126:233-239. [17] YU S, YU S, LIU H, et al. Enhancing mesenchymal stem cell survival and homing capability to improve cell engraftment efficacy for liver diseases. Stem Cell Res Ther. 2023;14(1):235. [18] HU C, LI L. Improvement of mesenchymal stromal cells and their derivatives for treating acute liver failure. J Mol Med (Berl). 2019;97(8): 1065-1084. [19] YU K, WANG Y, SUN H, et al. Silk Fibroin-Based Lenvatinib Nanomedicine with Conformation Tunability for Systemic Treatment of Hepatocellular Carcinoma. ACS Appl Mater Interfaces. 2024;16(44):60070-60083. [20] KATSELIS C, APOSTOLOU K, FERETIS T, et al. Role of Stem Cells Transplantation in Tissue Regeneration After Acute or Chronic Acetaminophen Induced Liver Injury. J Invest Surg. 2016;29(2):112-120. [21] YOU Y, WEN DG, GONG JP, et al. Research Status of Mesenchymal Stem Cells in Liver Transplantation. Cell Transplant. 2019;28(12):1490-1506. [22] 胡婧婧,何松霖,张大旭,等.基于脱细胞方法制备3D植物肝组织工程支架及表征[J].中国组织工程研究,2024,28(29):4645-4651. [23] HU J, HE S, ZHANG D, et al. Constructing liver-like tissue in situ based on plant-derived cellulose scaffolds alleviating acute liver injury. Mater Design. 2024;240:112856. [24] XU L, WANG S, SUI X, et al. Mesenchymal Stem Cell-Seeded Regenerated Silk Fibroin Complex Matrices for Liver Regeneration in an Animal Model of Acute Liver Failure. ACS Appl Mater Interfaces. 2017;9(17):14716-14723. [25] KIM MK, JEONG W, KANG HW. Liver dECM-Gelatin Composite Bioink for Precise 3D Printing of Highly Functional Liver Tissues. J Funct Biomater. 2023;14(8):417. [26] ZHANG L, YANG G, JOHNSON BN, et al. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019;84:16-33. [27] DADO D, LEVENBERG S. Cell-scaffold mechanical interplay within engineered tissue. Semin Cell Dev Biol. 2009;20(6):656-664. [28] KIM SH, HONG H, AJITERU O, et al. 3D bioprinted silk fibroin hydrogels for tissue engineering. Nat Protoc. 2021;16(12):5484-5532. [29] LEE YJ, LEE JS, AJITERU O, et al. Biocompatible fluorescent silk fibroin bioink for digital light processing 3D printing. Int J Biol Macromol. 2022;213:317-327. [30] ZHOU Z, CUI J, WU S, et al. Silk fibroin-based biomaterials for cartilage/osteochondral repair. Theranostics. 2022;12(11):5103-5124. [31] HUANG L, SHI J, ZHOU W, et al. Advances in Preparation and Properties of Regenerated Silk Fibroin. Int J Mol Sci. 2023;24(17):13153. [32] LI G, SUN S. Silk Fibroin-Based Biomaterials for Tissue Engineering Applications. Molecules. 2022;27(9):2757. [33] FAROKHI M, MOTTAGHITALAB F, REIS R L, et al. Functionalized silk fibroin nanofibers as drug carriers: Advantages and challenges. J Control Release. 2020;321:324-347. [34] WANI SUD, GAUTAM SP, QADRIE ZL, et al. Silk fibroin as a natural polymeric based bio-material for tissue engineering and drug delivery systems-A review. Int J Biol Macromol. 2020;163:2145-2161. [35] ZHANG D, FU L, YANG Y, et al. Tetrahedral framework nucleic acids improve the effectiveness of adipose-derived mesenchymal stem cells in the repair of acute liver failure. Mater Today Nano. 2024;25:100454. [36] KULKARNI AV, REDDY R, SHARMA M, et al. Healthcare utilization and outcomes of living donor liver transplantation for patients with APASL-defined acute-on-chronic liver failure. Hepatol Int. 2023; 17(5):1233-1240. [37] UCHIDA H, HONG SK, OKUMURA S, et al. Current Status and Outcomes of Living Donor Liver Transplantation for Pediatric Acute Liver Failure: Results From a Multicenter Retrospective Study Over Two Decades. Pediatr Transplant. 2024;28(6):e14838. [38] TAN Q, DENG S, XIONG L. Role of Kynurenine and Its Derivatives in Liver Diseases: Recent Advances and Future Clinical Perspectives. Int J Mol Sci. 2025;26(3):968. [39] VELAZQUEZ JJ, LEGRAW R, MOGHADAM F, et al. Gene Regulatory Network Analysis and Engineering Directs Development and Vascularization of Multilineage Human Liver Organoids. Cell Syst. 2021;12(1):41-55.e11. [40] WANG Y, KIM HJ, VUNJAK-NOVAKOVIC G, et al. Stem cell-based tissue engineering with silk biomaterials. Biomaterials. 2006;27(36): 6064-6082. [41] SAHOO JK, HASTURK O, FALCUCCI T, et al. Silk chemistry and biomedical material designs. Nat Rev Chem. 2023;7(5):302-318. [42] XIAO Y, QU Y, HU X, et al. E7 peptide modified poly(ε-caprolactone)/silk fibroin/octacalcium phosphate nanofiber membranes with “recruitment-osteoinduction” potentials for effective guided bone regeneration. Int J Biol Macromol. 2025;305(Pt 1):140862. [43] BI X, MAO Z, LI L, et al. Janus decellularized membrane with anisotropic cell guidance and anti-adhesion silk-based coatings for spinal dural repair. Nat Commun. 2025;16(1):1674. [44] TANG L, YANG Y, LI Y, et al. Knitted silk mesh-like scaffold incorporated with sponge-like regenerated silk fibroin/collagen I and seeded with mesenchymal stem cells for repairing Achilles tendon in rabbits. Acta Bioeng Biomech. 2018;20(4):77-87. [45] SUN W, GREGORY DA, TOMEH MA, et al. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int J Mol Sci. 2021;22(3):1499. [46] MU X, SAHOO JK, CEBE P, et al. Photo-Crosslinked Silk Fibroin for 3D Printing. Polymers (Basel). 2020;12(12):2936. [47] ZHANG X, LI W. [Research process of the preparation of electrostatic spinning of poly-glycerol sebacate and the application in tissue engineering]. Hua Xi Kou Qiang Yi Xue Za Zhi. 2015;33(5):539-542. [48] LI S, SHI X, XU B, et al. [Progress in the application of silk fibroin in tissue engineered drug delivery system]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2021;35(9): 1192-1199. [49] WU J, HONG Y. Enhancing cell infiltration of electrospun fibrous scaffolds in tissue regeneration. Bioact Mater. 2016;1(1):56-64. [50] KIM SH, KIM DY, LIM TH, et al. Silk Fibroin Bioinks for Digital Light Processing (DLP) 3D Bioprinting. Adv Exp Med Biol. 2020;1249:53-66. [51] LUTOLF MP, HUBBELL JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47-55. [52] VENKATESH SK, YIN M, EHMAN RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013;37(3):544-555. |
| [1] | Liu Yang, Liu Donghui , Xu Lei, Zhan Xu, Sun Haobo, Kang Kai. Role and trend of stimuli-responsive injectable hydrogels in precise myocardial infarction therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2072-2080. |
| [2] | Wang Qisa, Lu Yuzheng, Han Xiufeng, Zhao Wenling, Shi Haitao, Xu Zhe. Cytocompatibility of 3D printed methyl acrylated hyaluronic acid/decellularized skin hydrogel scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1912-1920. |
| [3] | Zhou Hongli, Wang Xiaolong, Guo Rui, Yao Xuanxuan, Guo Ru, Zhou Xiongtao, He Xiangyi. Fabrication and characterization of nanohydroxyapatite/sodium alginate/polycaprolactone/alendronate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1962-1970. |
| [4] | Bai Xiangyu, Huo Feng, Hao Yan, Wang Zecheng, Guo Xiaoyu. Platelet-derived growth factor BB-loaded chitosan/reduced graphene oxide scaffold for repairing alveolar bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 329-337. |
| [5] | Yuan Qian, Zhang Hao, Pang Jie. Characterization and biological properties of naringin-loaded chitosan/beta-tricalcium phosphate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 424-432. |
| [6] | Yang Fengli, Zhou Chao, Xiong Wei, Zhou Yuxiang, Li Dengshun, Wang Xin, Li Zhanzhen. 3D printed poly-L-lactic acid bone scaffolds in repair of bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 507-515. |
| [7] | Jiang Kan, Alimujiang·Abudourousuli, Shalayiding·Aierxiding, Aikebaierjiang·Aisaiti, Kutiluke·Shoukeer, Aikeremujiang·Muheremu. Biomaterials and bone regeneration: research hotspots and analysis of 500 influential papers [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 528-536. |
| [8] | Li Qian, Qumanguli · Abudukelimu, Shao Ziyu, Hu Yang. Hard template construction of nano-beta-tricalcium phosphate and nano-hydroxyapatite root canal sealing materials [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3597-3608. |
| [9] | Li Liang, Yang Han, Suo Hairui, Guan Lu, Wang Zhenlin. 3D printed methacrylated gelatin/chitosan scaffolds: evaluation of antibacterial, mechanical properties and cytocompatibility [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3636-3642. |
| [10] | Yang Lei, Liu Xinfang, Luo Sidong, Zhang Hongan, Wang Yeyang, Chen Weijian. Different preparation methods of silk fibroin and its application in the construction of small-diameter tissue-engineered blood vessels [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3694-3701. |
| [11] | Xiong Jiaying, Shen Jieyi, Lyu Jiahong. Characteristics and strategies of 3D-printed biomimetic bioceramic scaffolds for repairing jaw defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3709-3716. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||