Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (31): 6792-6799.doi: 10.12307/2025.552
Previous Articles Next Articles
Effect and mechanism of perinatal mesenchymal stem cells and their combination with hydrogels in treatment of intrauterine adhesions
Zhong Min1, 2, Wang Cheng2, 3, Fan Zhenhai1, 2, Li Linyan1, 2, Yu Limei2, 3, 4
- 1Department of Obstetrics and Gynecology (Prenatal Diagnostic Center), 2Guizhou Province Biofabrication Laboratory, 3Guizhou Province Center of Cell Engineering, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China; 4Collaborative Innovation Center of Tissue Damage Repair and Regenerative Medicine of Ministry of Education, Zunyi Medical University, Zunyi 563003, Guizhou Province, China
-
Received:2024-08-15Accepted:2024-09-23Online:2025-11-08Published:2025-02-27 -
Contact:Yu Limei, MD, Professor, Doctoral supervisor, Guizhou Province Biofabrication Laboratory, and Guizhou Province Center of Cell Engineering, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China; Collaborative Innovation Center of Tissue Damage Repair and Regenerative Medicine of Ministry of Education, Zunyi Medical University, Zunyi 563003, Guizhou Province, China -
About author:Zhong Min, Master candidate, Department of Obstetrics and Gynecology (Prenatal Diagnostic Center), and Guizhou Province Biofabrication Laboratory, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China -
Supported by:National Natural Science Foundation of China, No. 82060270 (to YLM); Zunyi Big Data and Science and Technology Bureau Municipal Institute Joint Fund Basic Project, No. [Zunyi Science and Technology Bureau HZ (2023) 282] (to LLY)
CLC Number:
Cite this article
Zhong Min, Wang Cheng, Fan Zhenhai, Li Linyan, Yu Limei. Effect and mechanism of perinatal mesenchymal stem cells and their combination with hydrogels in treatment of intrauterine adhesions[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6792-6799.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
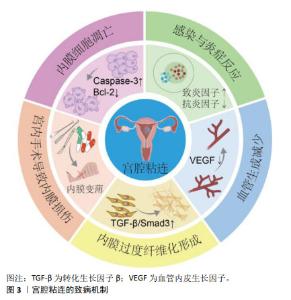
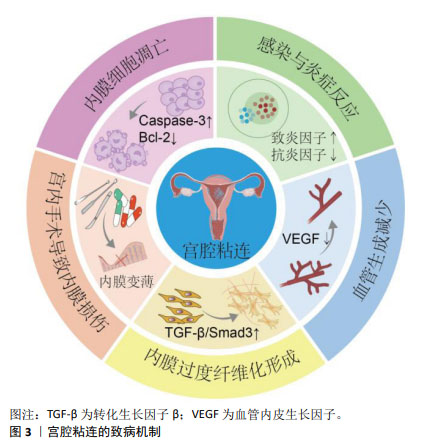
2.1 子宫内膜的结构与功能 子宫内膜是一种高度再生的组织,主要由上皮细胞、间质细胞、血管平滑肌细胞、内皮细胞和免疫细胞组成[25],分泌期子宫内膜的完整性是影响子宫内膜容受性与健康受精卵着床的决定性因素[26]。子宫内膜表面2/3的致密层和海绵层被统称为功能层,受垂体-下丘脑-卵巢轴激素的调节,内膜周期性增殖、分泌和剥脱,形成增殖期、分泌期、月经期3个阶段,形成月经的周期性出血[5];而内膜靠近子宫肌层的1/3为基底层,不受卵巢激素影响,含有丰富的子宫内膜干细胞,月经后可再生,达到无瘢痕、生理性修复子宫内膜创面,重新形成子宫内膜功能层[27-29]。各种原因造成子宫内膜损伤和纤维化瘢痕修复,是导致宫腔粘连的关键。 2.2 宫腔粘连发生机制 宫腔粘连又称为Asherman综合征,病理特点是子宫内膜损伤或先天发育异常导致子宫内膜发生纤维化,正常的激素敏感性子宫内膜被过度沉积的细胞外基质所替代,无血管、无功能的纤维粘连束增多,导致宫腔或宫颈阻塞[30]。既往研究认为子宫内膜干/祖细胞被认为是维持正常子宫内膜功能和形态所必需的细胞,内膜干细胞缺失是导致子宫内膜再生与功能障碍的重要原因[31-33]。宫腔粘连的致病机制涉及内膜细胞凋亡和基底干/祖细胞缺失、慢性炎症反应、血管新生受抑、上皮间质转化等,形成纤维状或致密粘连,产生子宫内膜纤维化瘢痕修复[9],见图3。宫腔粘连的病理表现为子宫内膜的上皮细胞间连接缺失、腺体缺乏、细胞外基质降解,大量上皮细胞发生间质转化,间质细胞标志物α-平滑肌肌动蛋白、成纤维细胞特异性蛋白1和波形蛋白显著上调,而内皮细胞特异性标志物CD31表达下调[34-35],内膜被纤维组织取代。内膜组织中免疫细胞持续浸润,持续较高水平释放肿瘤坏死因子α、白细胞介素1β和白细胞介素17等致炎因子,降低白细胞介素4和白细胞介素10等抗炎因子水平,激活转化生长因子β/Smad3信号途径,明显促进子宫内膜纤维化,而血管内皮生长因子、表皮生长因子、胰岛素样生长因子1等促修复因子减少,内膜细胞增殖和新生血管形成受抑制。因此,针对宫腔粘连的发生机制,寻找关键干预靶分子和细胞事件,将有益于防治宫腔粘连的发生、进展与复发。间充质干细胞治疗心肌梗死、肝和肺纤维化、自身免疫性疾病、卵巢功能不全等疾病中,已显示出促进损伤组织再生、抗纤维化、旁分泌、抗炎、免疫调节、抗凋亡等多种作用[18,36-39],提示间充质干细胞针对宫腔粘连的发病机制,可能发挥明显治疗作用。 "
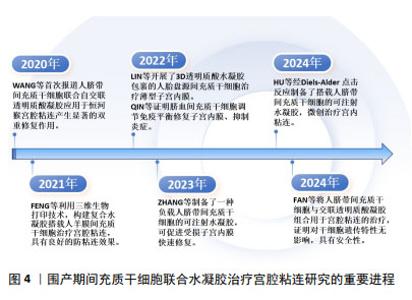
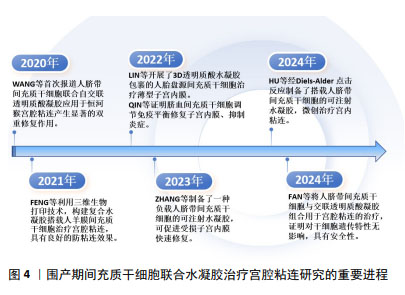
2.3 水凝胶在宫腔粘连防治中的应用 水凝胶是一种具有三维空间网络结构、亲水性新型功能高分子聚合物,主要由凝胶单体通过化学或物理交联形成[40]。水凝胶因高含水量、生物可降解性和生物相容性而被广泛用作细胞和生长因子的载体。天然大分子水凝胶主要由透明质酸、壳聚糖、明胶、胶原蛋白和纤维蛋白等聚合物组成,具有组织相容性好、可降解性好、易于细胞黏附和迁移、制备成本低等优点[41]。合成水凝胶主要由聚乙二醇、聚氧乙烯和聚丙烯酰胺等合成[42],在制备过程中,聚合物的浓度、机械强度和生物降解性均可调节,以适应使用环境[23]。水凝胶通过模拟天然细胞外基质作为结构模板,为细胞黏附、迁移、增殖、分化和维持生长提供了三维环境,为移植细胞更好地发挥治疗作用提供保障[43]。最新一项2 359例患者应用透明质酸钠凝胶治疗宫内手术后子宫腔粘连,对生育能力的影响及安全性评价的研究结果表明,透明质酸凝胶安全性良好,能显著降低宫腔粘连的发生率,并可能改善生育结局[44]。单独使用水凝胶治疗宫腔粘连中,水凝胶较好的生物相容性和三维网状结构及膨胀率,为内膜细胞迁移、黏附和保持宫腔形态提供了较好的支撑作用,但相对间充质干细胞强大的生长因子分泌功能而言,水凝胶缺乏强大的促内膜细胞再生及抑制上皮细胞间质转化的作用,水凝胶表面难以进行功能修饰,也不能像间充质干细胞一样适应局部变化,通过分泌功能发挥更强的治疗作用,因此,水凝胶在提供细胞增殖、分化所需的多种生长因子、核酸、多肽环境,及抗炎、免疫调节、旁分泌等作用方面存在明显不足,甚至有的水凝胶还存在韧性较差、强度低,作为支撑维持稳定性和时长不确定的局限[45]。ZHANG等[46]新开发了一种基于普鲁洛F127/F68和姜黄素的温敏性水凝胶,可有效提高姜黄素的溶解性和生物利用度,体内实验表明,与已有的传统的水凝胶相比,这种水凝胶能更好地促进子宫内膜再生,增加子宫内膜血液供应,减少纤维蛋白的异常沉积,从而更有效地预防宫腔粘连的形成。 2.4 不同来源的间充质干细胞在宫腔粘连中的应用 间充质干细胞阳性表达CD44、CD73、CD90和CD105,不表达CD34、CD45、CD19、CD11b和HLA-DR,具有自我更新和多向分化潜能,且来源丰富,除源于骨髓、脂肪组织外,脐带、羊膜、胎盘、脐血来源间充质干细胞被统称为围产期间充质干细胞。不同来源的间充质干细胞静脉滴注后通过损伤部位高水平趋化因子作用归巢到受损区域,原位注射也可直接发挥治疗作用,显著改善子宫内膜再生[40]。但无创宫腔内给予间充质干细胞,其在宫腔的驻留和作用维持却成了疗效不确定的问题。在无水乙醇、三氯乙酸、机械损伤及其联合脂多糖诱导的宫腔粘连治疗中,骨髓间充质干细胞静脉注射或宫内注射,都明显增加了子宫内膜厚度及容受性,减少了纤维化[47-50];较骨髓间充质干细胞治疗而言,骨髓间充质干细胞联合泊洛沙姆407水凝胶治疗机械损伤所致宫腔粘连,更明显地增强移植细胞的黏附性,更好地修复受损子宫内膜,改善子宫内膜组织结构,恢复生育功能[51]。应用经血间充质干细胞联合羊膜提取物水凝胶,也能更好地协同改善内膜再生环境,有效治疗损伤所致的宫腔粘连[52]。脂肪间充质干细胞衍生的外泌体联合水凝胶治疗宫腔粘连也显示了子宫内膜再生与生育能力恢复的效果[53]。骨髓、经血、羊膜、脐带和脐血来源间充质干细胞的治疗作用机制分别与调节Wnt/β-catenin、Hippo/TAZ、磷脂酰肌醇3-激酶(phosphatidylinositol 3 kinase,PI3K)/蛋白激酶B(protein kinase B,AKT)通路,促巨噬细胞向M2型极化,激活Notch信号、调节核因子κB及免疫平衡有关[18,50,54-57]。然而,尽管骨髓间充质干细胞是最早用于宫腔粘连的干细胞,但与脂肪间充质干细胞相似,有限的数量和有创采集等限制了他们的应用。近年来,围产期间充质干细胞无创采集、原始数量级高、免疫原性低等优势日益凸显,成为开发间充质干细胞治疗宫腔粘连关注的新焦点,围产期间充质干细胞联合水凝胶治疗宫腔粘连的重要进程如图4所示。"

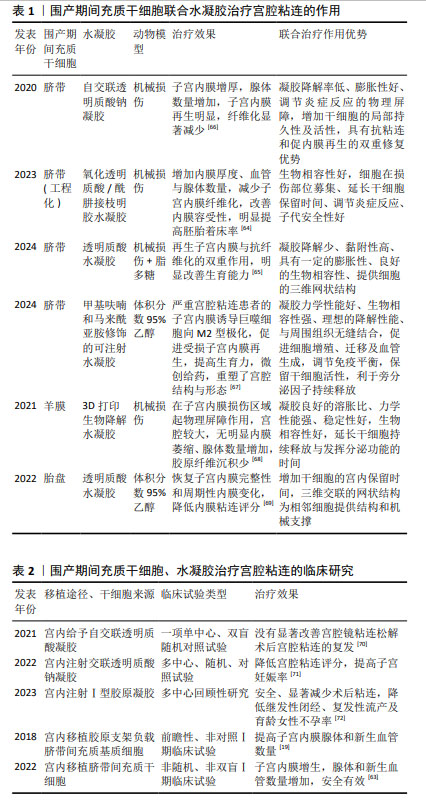
2.5 围产期间充质干细胞治疗宫腔粘连的作用和机制 2.5.1 脐带间充质干细胞及其联合水凝胶 与骨髓间充质干细胞相比,脐带间充质干细胞被广泛认为更具转化应用优势[58],具有低免疫原性、增殖能力强、取材简便、无创采集等优势,脐带为医疗废弃物,伦理限制较少,已逐渐成为干细胞治疗和类器官建立的重要种子资源,也是宫腔粘连治疗中研究最多的干细胞。QIN等[56]的研究报道,经褪黑激素预处理人的脐带间充质干细胞不仅调节巨噬细胞向M2型极化,减轻炎症反应,还促进内膜细胞增殖,减少内膜基质细胞凋亡,可上调人脐带间充质干细胞Oct-4 和SOX2干性基因表达,进而减少内膜纤维化病变,增加子宫内膜厚度、腺体和新生血管数量,增加胚胎着床数。脐带间充质干细胞培养上清液可通过激活Wnt5a/β-catenin信号通路,促进人子宫内膜细胞增殖和迁移,提示上调或激活Wnt5a可能是治疗宫腔粘连的一个潜在靶点[59]。脐带间充质干细胞也可通过激活Hippo/TAZ信号通路,抑制上皮间质转化;通过激活SDF-1/CXCR4轴,促进子宫内膜增殖,产生强大的再生受损子宫内膜的能力,恢复大鼠的生育力[60]。此外,脐带间充质干细胞衍生的外泌体中包含的miR-7162-3p可以通过靶向调节APOL6 mRNA表达,减少米非司酮诱导的子宫内膜基质细胞凋亡,揭示脐带间充质干细胞分泌外泌体及miRNA的靶向沉默效应产生提高内膜细胞存活的作用,可能是治疗子宫内膜损伤的一种无细胞治疗新策略[61]。脐带间充质干细胞来源的外泌体部分通过 miR-140-3p/FOXP1/Smad 轴减轻转化生长因子β1诱导的细胞纤维化,也进一步明确了间充质干细胞衍生外泌体抗纤维化治疗作用[62]。2022年,HUANG等[63]开展的10例子宫内膜损伤治疗临床研究中(宫腔粘连6例和剖宫产瘢痕憩室4例),将2 mL溶液重悬的细胞浓度为1×1010 L-1的临床级脐带间充质干细胞涂抹在导管上并置于子宫腔中,经过2次宫内移植,治疗6个月后子宫内膜厚度、体积及剖宫产瘢痕憩室有改善趋势,所有患者血常规、肝肾功能、卵巢功能、肿瘤生物标志物和免疫功能等血液生化检测均正常,胸部X射线片和心电图也未见异常,显示了脐带间充质干细胞宫内移植治疗的有效性和安全性。 采用负载脐带间充质干细胞的氧化透明质酸和酰肼接枝明胶组合局部注射[64]、脐带间充质干细胞联合交联透明质酸凝胶复合物植入宫腔[65],都显著增加子宫内膜的厚度、血管和腺体的数量,明显减轻子宫内膜纤维化,并通过激活 MEK/ERK1/2 信号通路,降低炎症因子白细胞介素1β和白细胞介素6水平,升高抗炎因子白细胞介素10水平,诱导子宫内膜血管内皮生长因子高表达,促进损伤的子宫内膜快速恢复,改善子宫内膜对胚胎的容受性,见表1。在非人灵长类动物恒河猴模型中,宫腔内联合给予自交联透明质酸凝胶和人脐带间充质干细胞,对损伤的子宫内膜具有防粘连和促进内膜再生的双重作用[66],且所制备的可注射水凝胶机械性能稳定,具有良好的生物相容性和适宜的降解性,能较好地适应于复杂的子宫环境,尤其为严重宫腔粘连患者提供了一种有前途的微创治疗策略。已开展的一些临床试验也初步验证了脐带间充质干细胞、水凝胶等生物材料治疗宫腔粘连的有效性和安全性,见表2。宫腔粘连松解术后将搭载1×107个脐带间充质干细胞的胶原支架植入患者的宫腔后,辅以激素调节内膜周期形成,治疗3个月后子宫内膜厚度增加,内膜雌激素受体和新生血管重要因子Ki67和血管性血友病因子表达均明显上调,表明子宫内膜增殖、腺体和新生血管形成均得到显著改善,子宫内膜容受性明显提高;26例重度子宫内膜粘连患者,术后30个月随访,已有10例患者怀孕,生育力明显恢复[19]。尽管交联透明质酸钠凝胶已在临床常规用于宫腔粘连治疗,但围产期间充质干细胞目前仅有脐带间充质干细胞进入了临床试验研究阶段。 "
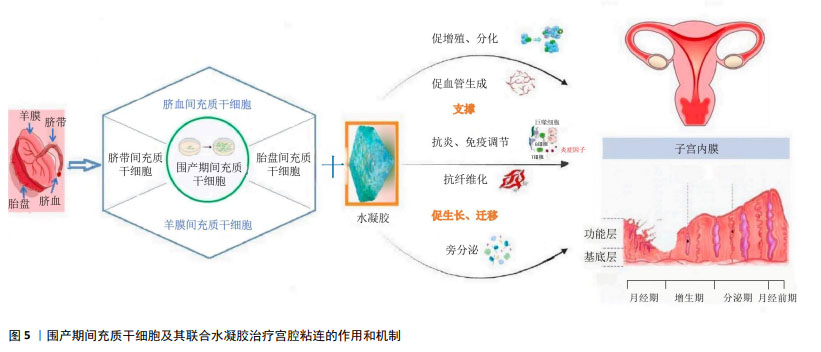
2.5.2 羊膜间充质干细胞及其联合水凝胶 附着于胎盘的人羊膜也是一种医疗废弃物,无创采集、伦理限制少。羊膜由上皮层、基底层和间质层组成,羊膜间充质干细胞源于羊膜间质层,来源丰富,分离操作简单、原始数量多是其优势。羊膜间充质干细胞的免疫原性低、无致瘤性,具有增殖力高和多向分化潜能强的优点,同为干细胞疗法的重要种子细胞[73-74]。羊膜间充质干细胞也表达波形蛋白、CD44、CD73、CD90和CD105及干性标志蛋白、血管性血友病因子、血小板/内皮细胞黏附分子、血管内皮生长因子、血管生成素1、内皮和血管生成标志物等[75]。近年的动物研究已显示,移植羊膜间充质干细胞是子宫内膜再生的有效替代方案,通过免疫调节、抗炎、分泌细胞因子和促进子宫内膜再生与血管生成等发挥治疗作用[76-77],作用机制涉及下调转化生长因子β1/Smad途径、上调血管内皮生长因子、诱导羊膜间充质干细胞分化为子宫内膜样细胞、与雌激素协同促进子宫内膜及腺体再生等。人羊膜间充质干细胞还可通过激活Notch信号通路,促进子宫内膜再生;也可过旁分泌作用或转分化为内膜细胞而促进子宫内膜修复[78]。利用三维生物打印技术构建含有不同比例甲基丙烯酸酯化明胶和甲基丙烯酸化胶原蛋白复合水凝胶与人羊膜间充质干细胞的混合物,不但具有良好的细胞相容性和稳定性[68],还保持了较强的机械性能,具有明显的防粘连作用,能延长干细胞的宫内持续释放时间,可长达7 d以上,宫腔内注射治疗后大鼠子宫腔增大、子宫内膜再生良好、纤维化面积明显减少。 2.5.3 胎盘间充质干细胞及其联合水凝胶 胎盘间充质干细胞源自常规被废弃的胎盘组织,伦理限制小。胎盘间充质干细胞具有脐带间充质干细胞相似的特征与功能,不仅具有强大的自我更新和多向分化潜能,还可分泌miRNA、外泌体和多种细胞因子发挥治疗多种疾病的作用。胎盘间充质干细胞也能分泌多种miRNA,如miR-125b-5p、miR-30c-5p 和 miR-23a-3p等,可通过抑制转化生长因子β/smad 信号通路,促进细胞增殖、逆转纤维化,恢复大鼠受损子宫功能,提高生育率[79]。小鼠实验表明,胎盘间充质干细胞联合透明质酸水凝胶能明显延长干细胞在薄型子宫内膜小鼠子宫中的保留时间,并通过增加子宫内膜厚度和腺体数量,减少纤维化面积、促进血管生成来修复子宫内膜,明显提高了胚胎着床率[69]。机制研究发现,胎盘间充质干细胞可通过激活 JNK/Erk1/2-Stat3-血管内皮生长因子通路促进人子宫内膜基质细胞的增殖和迁移,通过 Jak2-Stat5 和 c-Fos-血管内皮生长因子途径,促进腺细胞增殖,进而修复受损子宫内膜。 2.5.4 脐血间充质干细胞及其联合水凝胶 源于脐血的间充质干细胞,尽管存在体外培养成功率的问题,但仍是一种高活性、低免疫原性的间充质干细胞。给乙醇诱导子宫内膜损伤大鼠宫内移植脐血间充质干细胞,可明显增加受损子宫内膜厚度、腺体数量及促进血管内皮生长因子与增殖细胞核抗原的蛋白表达,其作用机制可能与PI3K/Akt信号通路激活有关[80]。尚有文献报道,脐血间充质干细胞移植治疗后,除子宫内膜修复较好外,内膜还高表达雌激素受体和 Ki-67 蛋白,纤维化率也显著降低,且可通过调节核因子κB 信号恢复Treg/Th17平衡,抑制免疫反应,减轻内膜炎症损伤;体外实验的单细胞mRNA测序结果显示,脐血间充质干细胞可以转分化为子宫内膜细胞[57],提示对于严重内膜细胞缺失的宫腔粘连而言,脐血间充质干细胞移植治疗可能更具优势。但脐血间充质干细胞联合水凝胶治疗宫腔粘连或子宫内膜损伤的研究尚未见报道,值得进一步探索。 围产期间充质干细胞及其联合水凝胶治疗宫腔粘连的作用和机制示意图,见图5。 "
| [1] ZHU X, PÉAULT B, YAN G, et al. Stem Cells and Endometrial Regeneration: From Basic Research to Clinical Trial. Curr Stem Cell Res Ther. 2019;14(4): 293-304. [2] ZHANG L, LI Y, DONG YC, et al. Transplantation of umbilical cord-derived mesenchymal stem cells promotes the recovery of thin endometrium in rats. Sci Rep. 2022;12(1):412. [3] EMINGR M, HALAJ M, MALČÁK M, et al. Prevention of intrauterine adhesions. Ceska Gynekol. 2023;88(3):210-213. [4] LIU T, HE B, XU X. Repairing and Regenerating Injured Endometrium Methods. Reprod Sci. 2023;30(6):1724-1736. [5] LV Q, WANG L, LUO X, et al. Adult stem cells in endometrial regeneration: Molecular insights and clinical applications. Mol Reprod Dev. 2021;88(6): 379-394. [6] WANG PH, YANG ST, CHANG WH, et al. Intrauterine adhesion. Taiwan J Obstet Gynecol. 2024;63(3):312-319. [7] DREISLER E, KJER JJ. Asherman’s syndrome: current perspectives on diagnosis and management. Int J Womens Health. 2019;11:191-198. [8] DI SPIEZIO-SARDO A, DE ANGELIS MC, DIMITRIOS K, et al. Restoring Fertility of Patients with Severe Asherman’s Syndrome in the Office Setting: A Step-by-Step Recipe for Success. J Minim Invasive Gynecol. 2023;30(5):355-356. [9] LI X, LV H F, ZHAO R, et al. Recent developments in bio-scaffold materials as delivery strategies for therapeutics for endometrium regeneration. Mater Today Bio. 2021;11:100101. [10] NIE N, GONG L, JIANG D, et al. 3D bio-printed endometrial construct restores the full-thickness morphology and fertility of injured uterine endometrium. Acta Biomater. 2023;157:187-199. [11] KHAN Z, GOLDBERG JM. Hysteroscopic Management of Asherman’s Syndrome. J Minim Invasive Gynecol. 2018;25(2):218-228. [12] HOU X, LIU Y, STREULI I, et al. Endometrial Regeneration in Asherman’s Syndrome: Clinical and Translational evidence of Stem Cell Therapies. Curr Stem Cell Res Ther. 2019;14(6):454-459. [13] PARASHAR S, PAJAI S, TARANG T. Recent Advancement in the Management of Intrauterine Adhesions Using Stem Cell Therapy: A Review Article. Cureus. 2023;15(8):e43553. [14] LI C, HU Y. Extracellular Vesicles Derived from Mesenchymal Stem Cells as Cell-Free Therapy for Intrauterine Adhesion. Int J Stem Cells. 2023;16(3): 260-268. [15] LIANG Y, SHUAI Q, ZHANG X, et al. Incorporation of Decidual Stromal Cells Derived Exosomes in Sodium Alginate Hydrogel as an Innovative Therapeutic Strategy for Advancing Endometrial Regeneration and Reinstating Fertility. Adv Healthc Mater. 2024;13(13):e2303674. [16] YANG JH, CHEN CD, CHEN SU, et al. The influence of the location and extent of intrauterine adhesions on recurrence after hysteroscopic adhesiolysis. Bjog. 2016;123(4):618-623. [17] ABUDUKEYOUMU A, LI M Q, XIE F. Transforming growth factor-β1 in intrauterine adhesion. Am J Reprod Immunol. 2020;84(2):e13262. [18] ZHANG S, ZHANG R, YIN X, et al. MenSCs Transplantation Improve the Viability of Injured Endometrial Cells Through Activating PI3K/Akt Pathway. Reprod Sci. 2023;30(11):3325-3338. [19] CAO Y, SUN H, ZHU H, et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther. 2018;9(1):192. [20] LIU F, HU S, YANG H, et al. Hyaluronic Acid Hydrogel Integrated with Mesenchymal Stem Cell-Secretome to Treat Endometrial Injury in a Rat Model of Asherman’s Syndrome. Adv Healthc Mater. 2019;8(14):e1900411. [21] KOŁODZIEJSKA M, JANKOWSKA K, KLAK M, et al. Chitosan as an Underrated Polymer in Modern Tissue Engineering. Nanomaterials (Basel). 2021;11(11):3019. [22] KARGOZAR S, GORGANI S, NAZARNEZHAD S, et al. Biocompatible Nanocomposites for Postoperative Adhesion: A State-of-the-Art Review. Nanomaterials (Basel). 2023;14(1):4. [23] RICHBOURG NR, WANCURA M, GILCHRIST AE, et al. Precise control of synthetic hydrogel network structure via linear, independent synthesis-swelling relationships. Sci Adv. 2021;7(7):eabe3245. [24] WU F, LEI N, YANG S, et al. Treatment strategies for intrauterine adhesion: focus on the exosomes and hydrogels. Front Bioeng Biotechnol. 2023;11: 1264006. [25] DE MIGUEL-GóMEZ L, LóPEZ-MARTíNEZ S, FRANCéS-HERRERO E, et al. Stem Cells and the Endometrium: From the Discovery of Adult Stem Cells to Pre-Clinical Models. Cells. 2021;10(3):595. [26] NEYKOVA K, TOSTO V, GIARDINA I, et al. Endometrial receptivity and pregnancy outcome. J Matern Fetal Neonatal Med. 2022;35(13):2591-2605. [27] COUSINS FL, FILBY CE, GARGETT CE. Endometrial Stem/Progenitor Cells-Their Role in Endometrial Repair and Regeneration. Front Reprod Health. 2021;3:811537. [28] GIRI J, MODI D. Endometrial and placental stem cells in successful and pathological pregnancies. J Assist Reprod Genet. 2023;40(7):1509-1522. [29] SALAMONSEN LA, HUTCHISON JC, GARGETT CE. Cyclical endometrial repair and regeneration. Development. 2021;148(17):dev199577. [30] CHEN G, LIU L, SUN J, et al. Foxf2 and Smad6 co-regulation of collagen 5A2 transcription is involved in the pathogenesis of intrauterine adhesion. J Cell Mol Med. 2020;24(5):2802-2818. [31] CHEN K, ZHENG S, FANG F. Endometrial Stem Cells and Their Applications in Intrauterine Adhesion. Cell Transplant. 2023;32:9636897231159561. [32] HONG IS. Endometrial stem/progenitor cells: Properties, origins, and functions. Genes Dis. 2023;10(3):931-947. [33] WEI X, LI H, CHEN T, et al. Histological study of telocytes in mice intrauterine adhesion model and their positive effect on mesenchymal stem cells in vitro. Cell Biol Int. 2024;48(5):647-664. [34] XU C, BAO M, FAN X, et al. EndMT: New findings on the origin of myofibroblasts in endometrial fibrosis of intrauterine adhesions. Reprod Biol Endocrinol. 2022;20(1):31. [35] LI J, DU S, SHENG X, et al. MicroRNA-29b Inhibits Endometrial Fibrosis by Regulating the Sp1-TGF-β1/Smad-CTGF Axis in a Rat Model. Reprod Sci. 2016;23(3):386-394. [36] CHEN JM, HUANG QY, ZHAO YX, et al. The Latest Developments in Immunomodulation of Mesenchymal Stem Cells in the Treatment of Intrauterine Adhesions, Both Allogeneic and Autologous. Front Immunol. 2021;12:785717. [37] MALDONADO VV, PATEL NH, SMITH EE, et al. Clinical utility of mesenchymal stem/stromal cells in regenerative medicine and cellular therapy. J Biol Eng. 2023;17(1):44. [38] SAAD-NAGUIB MH, KENFACK Y, SHERMAN LS, et al. Impaired receptivity of thin endometrium: therapeutic potential of mesenchymal stem cells. Front Endocrinol (Lausanne). 2023;14:1268990. [39] FERNáNDEZ-GARZA LE, BARRERA-BARRERA SA, BARRERA-SALDAñA HA. Mesenchymal Stem Cell Therapies Approved by Regulatory Agencies around the World. Pharmaceuticals (Basel). 2023;16(9):1334. [40] CAI J, GUO J, WANG S. Application of Polymer Hydrogels in the Prevention of Postoperative Adhesion: A Review. Gels. 2023;9(2):98. [41] D’AGOSTINO A, STELLAVATO A, CORSUTO L, et al. Is molecular size a discriminating factor in hyaluronan interaction with human cells? Carbohydr Polym. 2017;157:21-30. [42] WANG B, FENG C, DANG J, et al. Preparation of Fibroblast Suppressive Poly(ethylene glycol)-b-poly(l-phenylalanine)/Poly(ethylene glycol) Hydrogel and Its Application in Intrauterine Fibrosis Prevention. ACS Biomater Sci Eng. 2021;7(1):311-321. [43] PENNAROSSA G, ARCURI S, DE IORIO T, et al. Current Advances in 3D Tissue and Organ Reconstruction. Int J Mol Sci. 2021;22(2):830. [44] LUO Y, SUN Y, HUANG B, et al. Effects and safety of hyaluronic acid gel on intrauterine adhesion and fertility after intrauterine surgery: a systematic review and meta-analysis with trial sequential analysis of randomized controlled trials. Am J Obstet Gynecol. 2024;231(1):36-50.35. [45] GAO F, XU Z, LIANG Q, et al. Osteochondral Regeneration with 3D-Printed Biodegradable High-Strength Supramolecular Polymer Reinforced-Gelatin Hydrogel Scaffolds. Adv Sci (Weinh). 2019;6(15):1900867. [46] ZHANG W, HE Y, CHU Y, et al. Amorphous curcumin-based hydrogels to reduce the incidence of post-surgical intrauterine adhesions. Regen Biomater. 2024;11:rbae043. [47] JING Z, QIONG Z, YONGGANG W, et al. Rat bone marrow mesenchymal stem cells improve regeneration of thin endometrium in rat. Fertil Steril. 2014;101(2):587-594. [48] SALAMA NM, ZAGHLOL SS, MOHAMED HH, et al. Suppression of the inflammation and fibrosis in Asherman syndrome rat model by mesenchymal stem cells: histological and immunohistochemical studies. Folia Histochem Cytobiol. 2020;58(3):208-218. [49] YUAN L, CAO J, HU M, et al. Bone marrow mesenchymal stem cells combined with estrogen synergistically promote endometrial regeneration and reverse EMT via Wnt/β-catenin signaling pathway. Reprod Biol Endocrinol. 2022;20(1):121. [50] XIONG Z, MA Y, HE J, et al. Apoptotic bodies of bone marrow mesenchymal stem cells inhibit endometrial stromal cell fibrosis by mediating the Wnt/β-catenin signaling pathway. Heliyon. 2023;9(11):e20716. [51] 吕妍,关永格,宋阳,等.水凝胶搭载骨髓间充质干细胞治疗大鼠子宫内膜损伤[J].中国组织工程研究,2022,26(31):4940-4945. [52] HAO X, ZHANG S, LI P, et al. Amniotic membrane extract-enriched hydrogel augments the therapeutic effect of menstrual blood-derived stromal cells in a rat model of intrauterine adhesion. Biomater Adv. 2022;142:213165. [53] LIN J, WANG Z, HUANG J, et al. Microenvironment-Protected Exosome-Hydrogel for Facilitating Endometrial Regeneration, Fertility Restoration, and Live Birth of Offspring. Small. 2021;17(11):e2007235. [54] ZHU H, PAN Y, JIANG Y, et al. Activation of the Hippo/TAZ pathway is required for menstrual stem cells to suppress myofibroblast and inhibit transforming growth factor β signaling in human endometrial stromal cells. Hum Reprod. 2019;34(4):635-645. [55] YU J, ZHANG W, HUANG J, et al. Management of intrauterine adhesions using human amniotic mesenchymal stromal cells to promote endometrial regeneration and repair through Notch signalling. J Cell Mol Med. 2021; 25(23):11002-11015. [56] QIN W, WANG J, HU Q, et al. Melatonin-pretreated human umbilical cord mesenchymal stem cells improved endometrium regeneration and fertility recovery through macrophage immunomodulation in rats with intrauterine adhesions†. Biol Reprod. 2023;109(6):918-937. [57] HUA Q, ZHANG Y, LI H, et al. Human umbilical cord blood-derived MSCs trans-differentiate into endometrial cells and regulate Th17/Treg balance through NF-κB signaling in rabbit intrauterine adhesions endometrium. Stem Cell Res Ther. 2022;13(1):301. [58] DING DC, CHANG YH, SHYU WC, et al. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24(3):339-347. [59] WEI X, LIU F, ZHANG S, et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Conditioned Medium Promotes Human Endometrial Cell Proliferation through Wnt/β-Catenin Signaling. Biomed Res Int. 2022; 2022:8796093. [60] LIU Y, CAI J, LUO X, et al. Collagen Scaffold with Human Umbilical Cord Mesenchymal Stem Cells Remarkably Improves Intrauterine Adhesions in a Rat Model. Gynecol Obstet Invest. 2020;85(3):267-276. [61] SHI Q, WANG D, DING X, et al. Exosome-shuttled miR-7162-3p from human umbilical cord derived mesenchymal stem cells repair endometrial stromal cell injury by restricting APOL6. Arch Biochem Biophys. 2021;707: 108887. [62] SONG M, MA L, ZHU Y, et al. Umbilical cord mesenchymal stem cell-derived exosomes inhibits fibrosis in human endometrial stromal cells via miR-140-3p/FOXP1/Smad axis. Sci Rep. 2024;14(1):8321. [63] HUANG J, LI Q, YUAN X, et al. Intrauterine infusion of clinically graded human umbilical cord-derived mesenchymal stem cells for the treatment of poor healing after uterine injury: a phase I clinical trial. Stem Cell Res Ther. 2022;13(1):85. [64] ZHANG D, DU Q, LI C, et al. Functionalized human umbilical cord mesenchymal stem cells and injectable HA/Gel hydrogel synergy in endometrial repair and fertility recovery. Acta Biomater. 2023;167:205-218. [65] FAN J, XIE J, LIAO Y, et al. Human umbilical cord-derived mesenchymal stem cells and auto-crosslinked hyaluronic acid gel complex for treatment of intrauterine adhesion. Aging (Albany NY). 2024;16(7):6273-6289. [66] WANG L, YU C, CHANG T, et al. In situ repair abilities of human umbilical cord-derived mesenchymal stem cells and autocrosslinked hyaluronic acid gel complex in rhesus monkeys with intrauterine adhesion. Sci Adv. 2020;6(21):eaba6357. [67] HU S, DAI Y, XIN L, et al. Minimally invasive delivery of human umbilical cord-derived mesenchymal stem cells by an injectable hydrogel via Diels-Alder click reaction for the treatment of intrauterine adhesions. Acta Biomater. 2024;177:77-90. [68] FENG M, HU S, QIN W, et al. Bioprinting of a Blue Light-Cross-Linked Biodegradable Hydrogel Encapsulating Amniotic Mesenchymal Stem Cells for Intrauterine Adhesion Prevention. ACS Omega. 2021;6(36):23067-23075. [69] LIN Y, DONG S, YE X, et al. Synergistic regenerative therapy of thin endometrium by human placenta-derived mesenchymal stem cells encapsulated within hyaluronic acid hydrogels. Stem Cell Res Ther. 2022; 13(1):66. [70] ZHOU Q, SHI X, SARAVELOS S, et al. Auto-Cross-Linked Hyaluronic Acid Gel for Prevention of Intrauterine Adhesions after Hysteroscopic Adhesiolysis: A Randomized Controlled Trial. J Minim Invasive Gynecol. 2021;28(2):307-313. [71] CHEN H, XIONG W, ZENG Y, et al. Efficacy and safety of auto-cross-linked hyaluronic gel to prevent intrauterine adhesion after hysteroscopic electrosurgical resection: a multi-center randomized controlled trial. Ann Transl Med. 2022;10(22):1217. [72] LEE KB, CHON SJ, KIM S, et al. Using Type I Collagen Gel to Prevent Postoperative Intrauterine Adhesion: A Multicenter Retrospective Study. J Clin Med. 2023;12(11):3764. [73] ABBASI-KANGEVARI M, GHAMARI S H, SAFAEINEJAD F, et al. Potential Therapeutic Features of Human Amniotic Mesenchymal Stem Cells in Multiple Sclerosis: Immunomodulation, Inflammation Suppression, Angiogenesis Promotion, Oxidative Stress Inhibition, Neurogenesis Induction, MMPs Regulation, and Remyelination Stimulation. Front Immunol. 2019;10:238. [74] 王钰莹,余丽梅.制备人羊膜间充质干细胞制剂与质量控制过程中关键问题的思考[J].中国组织工程研究,2022,26(25):4016-4021. [75] GHAMARI SH, ABBASI-KANGEVARI M, TAYEBI T, et al. The Bottlenecks in Translating Placenta-Derived Amniotic Epithelial and Mesenchymal Stromal Cells Into the Clinic: Current Discrepancies in Marker Reports. Front Bioeng Biotechnol. 2020;8:180. [76] GAN L, DUAN H, XU Q, et al. Human amniotic mesenchymal stromal cell transplantation improves endometrial regeneration in rodent models of intrauterine adhesions. Cytotherapy. 2017;19(5):603-616. [77] MAO Y, YANG Y, SUN C, et al. Human amniotic mesenchymal stem cells promote endometrium regeneration in a rat model of intrauterine adhesion. Cell Biol Int. 2023;47(1):75-85. [78] HUANG X, YANG X, HUANG J, et al. Human amnion mesenchymal stem cells promote endometrial repair via paracrine, preferentially than transdifferentiation. Cell Commun Signal. 2024;22(1):301. [79] LIU H, ZHANG X, ZHANG M, et al. Mesenchymal Stem Cell Derived Exosomes Repair Uterine Injury by Targeting Transforming Growth Factor-β Signaling. ACS Nano. 2024;18(4):3509-3519. [80] 王红红,刘静,骆硕,等.脐血间充质干细胞对薄型子宫内膜PI3K/Akt信号通路影响的实验研究[J].中国优生与遗传杂志,2022,30(8): 1342-1347. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Li Kaiying, Wei Xiaoge, Song Fei, Yang Nan, Zhao Zhenning, Wang Yan, Mu Jing, Ma Huisheng. Mechanism of Lijin manipulation regulating scar formation in skeletal muscle injury repair in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1600-1608. |
| [3] | Yu Jingbang, Wu Yayun. Regulatory effect of non-coding RNA in pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1659-1666. |
| [4] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [5] | Hu Taotao, Liu Bing, Chen Cheng, Yin Zongyin, Kan Daohong, Ni Jie, Ye Lingxiao, Zheng Xiangbing, Yan Min, Zou Yong. Human amniotic mesenchymal stem cells overexpressing neuregulin-1 promote skin wound healing in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1343-1349. |
| [6] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [7] | Li Dijun, Jiu Jingwei, Liu Haifeng, Yan Lei, Li Songyan, Wang Bin. Three-dimensional gelatin microspheres loaded human umbilical cord mesenchymal stem cells for chronic tendinopathy repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1356-1362. |
| [8] | Lou Guo, Zhang Min, Fu Changxi. Exercise preconditioning for eight weeks enhances therapeutic effect of adipose-derived stem cells in rats with myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1363-1370. |
| [9] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [10] | Huang Ting, Zheng Xiaohan, Zhong Yuanji, Wei Yanzhao, Wei Xufang, Cao Xudong, Feng Xiaoli, Zhao Zhenqiang. Effects of macrophage migration inhibitory factor on survival, proliferation, and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1380-1387. |
| [11] | Aikepaer · Aierken, Chen Xiaotao, Wufanbieke · Baheti. Osteogenesis-induced exosomes derived from human periodontal ligament stem cells promote osteogenic differentiation of human periodontal ligament stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1388-1394. |
| [12] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [13] | Xie Liugang, Cui Shuke, Guo Nannan, Li Aoyu, Zhang Jingrui. Research hotspots and frontiers of stem cells for Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1475-1485. |
| [14] | Li Jialin, Zhang Yaodong, Lou Yanru, Yu Yang, Yang Rui. Molecular mechanisms underlying role of mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1512-1522. |
| [15] | Sun Yuting, Wu Jiayuan, Zhang Jian. Physical factors and action mechanisms affecting osteogenic/odontogenic differentiation of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1531-1540. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||