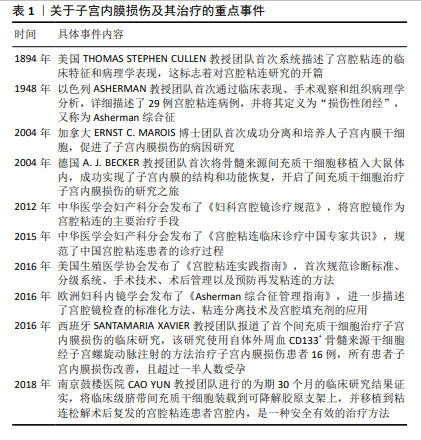
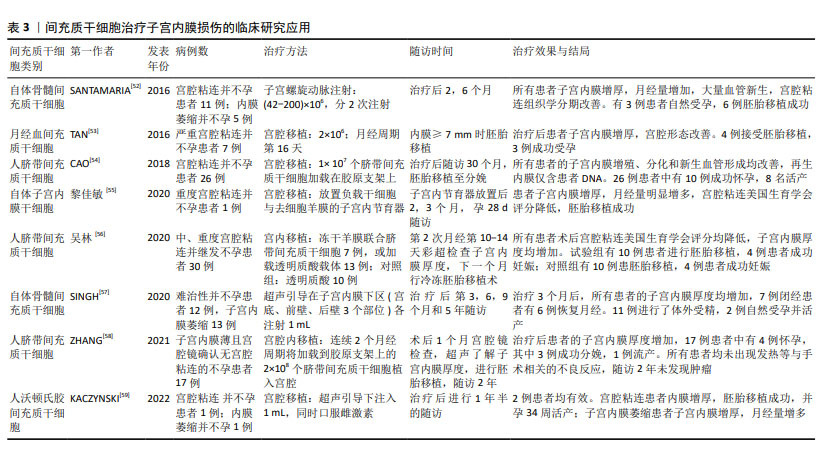
Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (31): 6782-6791.doi: 10.12307/2025.660
Previous Articles Next Articles
Mesenchymal stem cells and extracellular vesicles in repair of endometrial injury
Xiong Zhenghua1, 2, Zhou Jianghong2, Shen Yi2, Han Xuesong2
- 1Department of Gynecology of First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China; 2Department of Gynecology of Yan’an Hospital Affiliated to Kunming Medical University/Yan’an Hospital of Kunming City, Kunming 650051, Yunnan Province, China
-
Received:2024-07-04Accepted:2024-08-12Online:2025-11-08Published:2025-02-27 -
Contact:Han Xuesong, MD, Chief physician, Professor, Doctoral supervisor, Department of Gynecology of Yan’an Hospital Affiliated to Kunming Medical University/Yan’an Hospital of Kunming City, Kunming 650051, Yunnan Province, China -
About author:Xiong Zhenghua, Doctoral candidate, Department of Gynecology of First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China; Department of Gynecology of Yan’an Hospital Affiliated to Kunming Medical University/Yan’an Hospital of Kunming City, Kunming 650051, Yunnan Province, China -
Supported by:National Natural Science Foundation of China, No. 81960269 (to HXS); Yunnan Province “Ten Thousand People Plan” Famous Doctor Special Project, No. RLHXS20210331 (to HXS); Yunnan Province Medical Leadership Talent Training Program Project, No. L-2019005 (to HXS); Special Key Project of Yunnan Provincial Science and Technology Department-Kunming Medical University Joint, No. 202401AY070001-374 (to HXS); Scientific Research Fund Project of Yunnan Provincial Education Department, No. 2024Y242 (to XZH)
CLC Number:
Cite this article
Xiong Zhenghua, Zhou Jianghong, Shen Yi, Han Xuesong . Mesenchymal stem cells and extracellular vesicles in repair of endometrial injury[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6782-6791.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
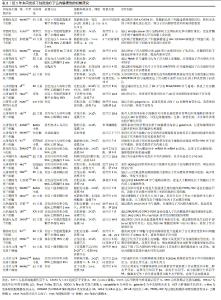
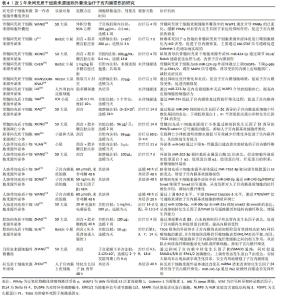
2.1 子宫内膜损伤的发病机制 子宫内膜组织由表浅的功能层和深部的基底层组成,功能层随着月经周期发生周期性的增厚和脱落,而基底层则负责再生新的功能层。子宫内膜损伤是妇科常见疾病,但发病机制未完全明确。任何原因导致的内膜基底层纤维化、细胞分化异常或障碍、血管生成受阻、内膜组织瘢痕形成等,均可引起宫腔粘连的发生[5]。研究表明,宫腔操作是子宫内膜损伤的主要原因,94.3%的宫腔粘连患者有宫腔操作,若流产≥3次则宫腔粘连风险增加4.6倍[6]。此外,炎症和感染、年龄、遗传因素、阴道菌群、宫腔微生态等也会影响宫腔粘连的发生。 2.1.1 子宫内膜纤维化 现有研究已证实纤维细胞增生异常活跃及内膜纤维化是宫腔粘连的主要发病机制。转化生长因子β具有调控细胞增殖、分化、凋亡等功能,是经典的促纤维化因子,在宫腔粘连患者子宫内膜组织中高表达,且随着宫腔粘连严重程度的增加其表达水平更高[7]。研究发现,转化生长因子β1通过激活经典的转化生长因子β/Smads通路和非经典的磷脂酰肌醇3-激酶/蛋白激酶B(phosphoinositide 3-kinase/protein kinase B,PI3K/AKT)通路、丝裂原活化蛋白激酶(mitogen-activated protein kinase pathway,MAPK)通路等多条信号通路发挥促纤维化作用,而转化生长因子β1的促纤维化作用受性激素、细胞因子和miRNAs的调控[8]。XUE等[9]研究表明,初始子宫内膜损伤后Wnt/β-catenin信号通路的瞬时激活促进了血管生成并增加了腺体的数量,有助于组织再生,而长时间的激活可能与纤维化形成相关。此外,基质金属蛋白酶9的表达降低会引起细胞外基质降解不足进而堆积,出现器官或组织纤维化,而细胞外基质的增加能促进转化生长因子β的合成[10]。基质金属蛋白酶9在宫腔粘连患者子宫内膜组织中低表达,且随着粘连程度的加重而降低,经药物或手术治疗后其表达升高[11]。还有研究表明,子宫内膜纤维化过程中存在Wnt/β-catenin通路与转化生长因子β/Smad通路间的相互作用,通过调节Wnt/β-catenin通路和细胞外基质的形成,能够有效抑制转化生长因子β对人子宫内膜基质细胞的促纤维化作用[7]。另外,血管生成是组织损伤修复的基础,宫腔粘连患者的血液和子宫内膜组织中血管内皮生长因子表达降低,经手术或药物治疗后血管内皮生长因子表达上调,纤维化减轻[12]。 2.1.2 子宫内膜干细胞分化异常 人子宫内膜干细胞首次于20世纪中叶被提出,并在1978-1989年间被两位学者修正,然而直到2004年才从子宫内膜组织中分离出来[13]。随后,有研究证实了子宫内膜干细胞具有促进子宫内膜再生和修复的功能,且当子宫内膜基底层受损时,子宫内膜干细胞出现数量减少或功能抑制,改变子宫内膜容受性,影响细胞归巢和迁移到受损部位,导致子宫内膜出现病理性修复,逐渐被纤维组织所替代,最终形成宫腔粘连[14]。 近年来,可检索到使用骨髓、月经血、脐带等来源的成体干细胞治疗重度子宫内膜损伤患者的个案及临床研究报道,近期疗效包括增加子宫内膜、改善月经情况和妊娠结局等[15],但远期疗效如产后的月经状况、子代智力及发育情况等则仍需进一步的追踪。 2.1.3 雌激素受体表达异常 女性的月经周期依赖于下丘脑-垂体-卵巢轴的调节,体内雌激素通过结合雌激素受体促进子宫内膜增殖和细胞分化。有研究报道,宫腔粘连患者子宫内膜组织中雌激素受体的表达明显升高,且具有月经周期性[16-17]。但也有研究报道,宫腔粘连患者子宫内膜组织中雌激素受体蛋白和mRNA均呈低表达,且重度宫腔粘连患者体内表达水平最低[18-19]。在临床诊治过程中发现,大多数重度宫腔粘连或顽固性子宫内膜过薄的患者,存在雌激素治疗不敏感的情况,补充雌激素后仍面临着子宫内膜再生障碍的难题,因此推测宫腔粘连患者可能存在雌激素受体分布异常或功能障碍,尚需要进一步的大样本数据进行论证。 2.1.4 其他 近年来,有学者从非编码RNA、转录、自噬、凋亡等方面进行子宫内膜损伤的发病机制研究。ZHANG等[20]研究表明,ADIRF-AS1、LINC00632、DIO3OS、MBNL1-AS1、MIR1-1HG-AS1、AC100803.2等关键长链非编码RNA参与了宫腔粘连的发展,并提出环磷酸鸟苷/蛋白激酶G (cyclic guanosine monophosphate/protein kinase G,cGMP/PKG)信号通路和离子转运可能是宫腔粘连发病机制研究的新方向。ZHOU等[21]研究证明了宫腔粘连患者的子宫内膜存在自噬缺陷,其通过碘代胞嘧啶二型碘化物脱碘酶2-丝裂原活化蛋白激酶/细胞外信号调节激酶-哺乳动物雷帕霉素靶蛋白途径(deiodinase 2-mitogen-activated protein kinase/extracellular signal-regulated kinase-mammalian target of rapamycin,DIO2-MAPK/ERK-MTOR)在子宫内膜上皮细胞-上皮间质转化中发挥重要作用。CHEN等[22]的大鼠体内研究表明,叉头框转录因子F2(Forkhead box F2,FOXF2)与Smad6相互作用并在宫腔粘连发病机制中共同调节COL5A2的转录表达,促进合成V型胶原,从而造成胶原沉积和宫腔粘连形成。 2.2 间充质干细胞在子宫内膜损伤修复中的研究进展 在中国知网、中华医学全文网、PubMed数据库筛选出近5年内关于间充质干细胞治疗子宫内膜损伤的机制研究有37篇文献,包括间充质干细胞治疗子宫内膜损伤的临床研究8篇。根据间充质干细胞的来源进行分类,将研究结果中所涉及的实验对象、造模方法、细胞疗法、作用机制等内容进行了总结[23-51],见表2。关于间充质干细胞治疗子宫内膜损伤的分子机制主要包括免疫调节、抗炎、抗纤维化、促进血管生成和组织再生等,涉及的信号通路有基质细胞源性因子1/C-X-C趋化因子受体4(stromal cell-derived factor-1/C-X-C chemokine receptor type 4,SDF-1/CXCR4)信号通路、Wnt/β-catenin信号通路、转化生长因子β/Smad信号通路、Notch信号通路、肝细胞生长因子/c-Met (hepatocyte growth factor/cMet,HGF/cMet)信号通路、Janus激酶/信号转导和转录激活因子3 (Janus kinase/signal transducer and activator of transcription 3,JAK/STAT3)信号通路、丝裂原活化蛋白激酶/细胞外信号调节激酶1/2(mitogen-activated protein kinase kinase/extracellular signal- regulated kinase 1/2,MEK/ERK1/2)信号通路、RhoA/Rho相关蛋白激酶I(RhoA/Rho-associated protein kinase I,RhoA/ROCKI)信号通路、IκB-α/核因子κB (IκB-α/nuclear factor kappa B,IκB-α/NF-κB)信号通路等[23-31]。间充质干细胞可能同时通过多种作用机制进行子宫内膜的损伤修复。首先,间充质干细胞具有显著的免疫调节功能,不仅能调控细胞归巢、自噬和铁死亡,还能分泌多种细胞因子和生长因子,减少炎细胞浸润并上调抗炎因子水平,减轻子宫内膜的炎症反应,调节子宫内膜的免疫微环境,进而促进内膜修复和再生[32-39]。间充质干细胞分泌的血管内皮生长因子、成纤维细胞生长因子等能促进血管新生,改善受损子宫内膜的血液供应,减轻或抑制子宫内膜纤维化[31-32]。其次,间充质干细胞还具有多向分化潜能,能够在特定微环境下分化为内膜上皮细胞、成纤维细胞等多种细胞类型,直接参与受损组织的修复[40-41]。最后,间充质干细胞通过旁分泌作用,释放多种活性分子,促进内膜基质和上皮的再生[42-44]。间充质干细胞分泌基质金属蛋白酶及其抑制剂,调节细胞外基质重塑,改善子宫内膜氧化应激过程和宫腔容受性,促进子宫内膜的修复和功能恢复[45-51]。 "

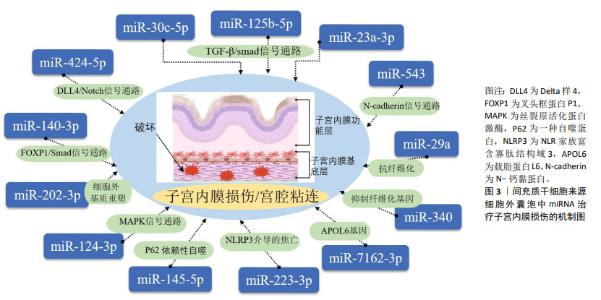
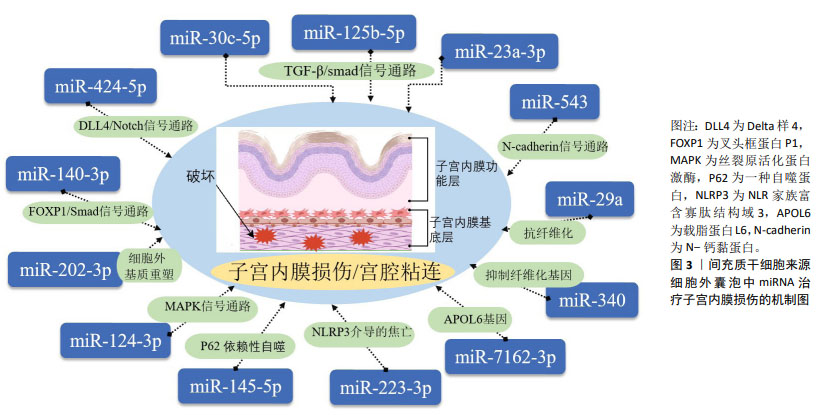
2.3 细胞外囊泡在子宫内膜损伤修复中的研究进展 在中国知网、中华医学全文网、PubMed 数据库筛选出近5年内涉及间充质干细胞衍生细胞外囊泡治疗子宫内膜损伤的相关研究19篇[60-79],见表4。动物研究结果显示,细胞外囊泡能够显著促进子宫内膜的再生和功能恢复,其治疗机制主要集中在对外泌体的研究,分析其原因可能是外泌体具有更明确的分泌途径和生物合成机制,其作为细胞间通讯的重要媒介,自被发现以来引起了广泛的关注。现有的研究表明,外泌体通过miRNA靶向调控子宫内膜组织中的纤维化相关因子、血管生成相关因子和细胞程序性死亡的效应因子,抑制子宫内膜纤维化,促进细胞增殖和血管生成,完成子宫内膜的损伤修复,具体miRNA及其靶基因作用机制见图3。值得注意的是,少量研究表明间充质干细胞来源的凋亡小体能通过Wnt/β-catenin信号通路抑制子宫内膜纤维化,促进子宫内膜再生,提高大鼠生育能力,且装载到透明质酸水凝胶后的疗效更好[68-69],但具体发挥作用的分子及其相应靶基因尚未明确,还需进一步的探索和研究。间充质干细胞衍生的细胞外囊泡在子宫内膜损伤修复中展现了广阔的应用前景,但需进一步研究验证其在临床中的安全性和有效性。 "

| [1] ANG CJ, SKOKAN TD, MCKINLEY KL. Mechanisms of Regeneration and Fibrosis in the Endometrium. Annu Rev Cell Dev Biol. 2023;39:197-221. [2] 中华医学会妇产科学分会.宫腔粘连临床诊疗中国专家共识[J].中华妇产科杂志,2015,50(12):881-887. [3] YU H, HUANG Y, YANG L. Research progress in the use of mesenchymal stem cells and their derived exosomes in the treatment of osteoarthritis. Ageing Res Rev. 2022;80:101684. [4] CHENG L, HILL AF. Therapeutically harnessing extracellular vesicles. Nat Rev Drug Discov. 2022;21(5):379-399. [5] LEE WL, LIU CH, CHENG M, et al. Focus on the Primary Prevention of Intrauterine Adhesions: Current Concept and Vision. Int J Mol Sci. 2021;22(10):5175. [6] SEVINÇ F, OSKOVI-KAPLAN ZA, ÇELEN Ş, et al. Identifying the risk factors and incidence of Asherman Syndrome in women with post-abortion uterine curettage. J Obstet Gynaecol Res. 2021;47(4):1549-1555. [7] LIU L, CHEN G, CHEN T, et al. si-SNHG5-FOXF2 inhibits TGF-β1-induced fibrosis in human primary endometrial stromal cells by the Wnt/β-catenin signalling pathway. Stem Cell Res Ther. 2020;11(1):479. [8] ABUDUKEYOUMU A, LI MQ, XIE F. Transforming growth factor-β1 in intrauterine adhesion. Am J Reprod Immunol. 2020;84(2):e13262. [9] XUE X, LI X, YAO J, et al. Transient and Prolonged Activation of Wnt Signaling Contribute Oppositely to the Pathogenesis of Asherman’s Syndrome. Int J Mol Sci. 2022;23(15):8808. [10] ESPINDOLA MS, HABIEL DM, COELHO AL, et al. Differential Responses to Targeting Matrix Metalloproteinase 9 in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2021;203(4):458-470. [11] LI C, WANG W, SUN S, et al. Expression and Potential Role of MMP-9 in Intrauterine Adhesion. Mediators Inflamm. 2021;2021:6676510. [12] WU F, LEI N, YANG S, et al. Treatment strategies for intrauterine adhesion: focus on the exosomes and hydrogels. Front Bioeng Biotechnol. 2023;11:1264006. [13] CHEN K, ZHENG S, FANG F. Endometrial Stem Cells and Their Applications in Intrauterine Adhesion. Cell Transplant. 2023;32: 9636897231159561. [14] SONG YT, LIU PC, TAN J, et al. Stem cell-based therapy for ameliorating intrauterine adhesion and endometrium injury. Stem Cell Res Ther. 2021;12(1):556. [15] ZHU X, PÉAULT B, YAN G, et al. Stem Cells and Endometrial Regeneration: From Basic Research to Clinical Trial. Curr Stem Cell Res Ther. 2019;14(4):293-304. [16] 赵淑芬,柳怡,郑雪湘.宫腔粘连患者子宫内膜组织中雌激素受体及孕激素受体的表达情况和临床意义分析[J].中国计划生育和妇产科,2018,10(7):33-36,41. [17] 陈庆,赵金燕,张雪,等.雌、孕激素通过TGF-β影响宫腔粘连的形成[J].山西医科大学学报,2021,52(7):883-888. [18] GE J, CHEN Y, YANG H, et al. Expression and significance of estrogen receptor and progesterone receptor in endometrial tissue of patients with intrauterine adhesions. Gland Surg. 2021;10(4):1478-1486. [19] 杜娟,康卉娴.ER和TGF-β1在宫腔粘连患者中的表达及其相关性的研究[J].宁夏医学杂志,2020,42(7):628-630. [20] ZHANG J, JIANG P, TU Y, et al. Identification and validation of long non-coding RNA associated ceRNAs in intrauterine adhesion. Bioengineered. 2022;13(1):1039-1048. [21] ZHOU Z, WANG H, ZHANG X, et al. Defective autophagy contributes to endometrial epithelial-mesenchymal transition in intrauterine adhesions. Autophagy. 2022;18(10):2427-2442. [22] CHEN G, LIU L, SUN J, et al. Foxf2 and Smad6 co-regulation of collagen 5A2 transcription is involved in the pathogenesis of intrauterine adhesion. J Cell Mol Med. 2020;24(5):2802-2818. [23] WANG Z, XIA L, CHENG J, et al. Combination Therapy of Bone Marrow Mesenchymal Stem Cell Transplantation and Electroacupuncture for the Repair of Intrauterine Adhesions in Rats: Mechanisms and Functional Recovery. Reprod Sci. 2024;31(8):2318-2330. [24] YUAN L, CAO J, HU M, et al. Bone marrow mesenchymal stem cells combined with estrogen synergistically promote endometrial regeneration and reverse EMT via Wnt/β-catenin signaling pathway. Reprod Biol Endocrinol. 2022;20(1):121. [25] YU J, ZHANG W, HUANG J, et al. Management of intrauterine adhesions using human amniotic mesenchymal stromal cells to promote endometrial regeneration and repair through Notch signalling. J Cell Mol Med. 2021;25(23):11002-11015. [26] SUN D, JIANG Z, CHEN Y, et al. MiR-455-5p upregulation in umbilical cord mesenchymal stem cells attenuates endometrial injury and promotes repair of damaged endometrium via Janus kinase/signal transducer and activator of transcription 3 signaling. Bioengineered. 2021;12(2):12891-12904. [27] ZHANG D, DU Q, LI C, et al. Functionalized human umbilical cord mesenchymal stem cells and injectable HA/Gel hydrogel synergy in endometrial repair and fertility recovery. Acta Biomater. 2023;167: 205-218. [28] LI J, HUANG B, DONG L, et al. WJ‑MSCs intervention may relieve intrauterine adhesions in female rats via TGF‑β1‑mediated Rho/ROCK signaling inhibition. Mol Med Rep. 2021;23(1):8. [29] HUA Q, ZHANG Y, LI H, et al. Human umbilical cord blood-derived MSCs trans-differentiate into endometrial cells and regulate Th17/Treg balance through NF-κB signaling in rabbit intrauterine adhesions endometrium. Stem Cell Res Ther. 2022;13(1):301. [30] 勾亚婷,张文文,李长江,等.NF-κB信号通路在人羊膜间充质干细胞治疗宫腔粘连中的作用[J].第三军医大学学报,2020,42(11): 1101-1108. [31] ZHOU L, WANG H, SHEN D, et al. Stem cells implanted with nanofibrous mats for injured endometrial regeneration and immune-microenvironment remodeling. Mater Today Bio. 2023;23:100855. [32] PARK M, HONG SH, PARK SH, et al. Perivascular Stem Cell-Derived Cyclophilin A Improves Uterine Environment with Asherman’s Syndrome via HIF1α-Dependent Angiogenesis. Mol Ther. 2020;28(8):1818-1832. [33] HU S, DAI Y, XIN L, et al. Minimally invasive delivery of human umbilical cord-derived mesenchymal stem cells by an injectable hydrogel via Diels-Alder click reaction for the treatment of intrauterine adhesions. Acta Biomater. 2024;177:77-90. [34] JIANG Q, LI J, PAN Y, et al. Melatonin-Primed MSCs Alleviate Intrauterine Adhesions by Affecting MSC-Expressed Galectin-3 on Macrophage Polarization. Stem Cells. 2022;40(10):919-931. [35] WANG J, LI J, YIN L, et al. MSCs promote the efferocytosis of large peritoneal macrophages to eliminate ferroptotic monocytes/macrophages in the injured endometria. Stem Cell Res Ther. 2024; 15(1):127. [36] MAO Y, YANG Y, SUN C, et al. Human amniotic mesenchymal stem cells promote endometrium regeneration in a rat model of intrauterine adhesion. Cell Biol Int. 2023;47(1):75-85. [37] 任莉,张俊俊,孟喜燕.人胎盘间充质干细胞对大鼠子宫内膜损伤的修复作用及对JAK2/STAT3通路的影响[J].中国实用医刊, 2023,50(1):1-5. [38] LIN Y, DONG S, YE X, et al. Synergistic regenerative therapy of thin endometrium by human placenta-derived mesenchymal stem cells encapsulated within hyaluronic acid hydrogels. Stem Cell Res Ther. 2022;13(1):66. [39] 郝赛楠,夏良君,席瑾,等.电针联合骨髓间充质干细胞移植通过调节SDF-1/CXCR4轴促进薄型子宫内膜修复的机制研究[J].针刺研究,2023,48(9):870-880. [40] WANG G, REN C, JIANG J. Effects of bone marrow mesenchymal stem cells on repair and receptivity of damaged endometrium in rats. J Obstet Gynaecol Res. 2021;47(9):3223-3231. [41] WANG L, YU C, CHANG T, et al. In situ repair abilities of human umbilical cord-derived mesenchymal stem cells and autocrosslinked hyaluronic acid gel complex in rhesus monkeys with intrauterine adhesion. Sci Adv. 2020;6(21):eaba6357. [42] XU X, XING Q, LIU R, et al. Therapeutic Effects and Repair Mechanism of HGF Gene-Transfected Mesenchymal Stem Cells on Injured Endometrium. Stem Cells Int. 2022;2022:5744538. [43] ZHANG L, LI Y, DONG YC, et al. Transplantation of umbilical cord-derived mesenchymal stem cells promotes the recovery of thin endometrium in rats. Sci Rep. 2022;12(1):412. [44] HUANG X, YANG X, HUANG J, et al. Human amnion mesenchymal stem cells promote endometrial repair via paracrine, preferentially than transdifferentiation. Cell Commun Signal. 2024;22(1):301. [45] WU M, LI Y, WANG Y, et al. HOXA10 Expressing UCMSCs Transplantation Improved Endometrial Receptivity on Endometrial Injury. Curr Stem Cell Res Ther. 2023;18(7):1001-1012. [46] FAN J, XIE J, LIAO Y, et al. Human umbilical cord-derived mesenchymal stem cells and auto-crosslinked hyaluronic acid gel complex for treatment of intrauterine adhesion. Aging (Albany NY). 2024;16(7):6273-6289. [47] XU X, KONG DS, TIAN YP, et al. Autocross-linked hyaluronic acid gel and adipose-derived mesenchymal stem cell composites for the treatment intrauterine adhesions. Taiwan J Obstet Gynecol. 2021;60(6): 1031-1037. [48] SHAO X, AI G, WANG L, et al. Adipose-derived stem cells transplantation improves endometrial injury repair. Zygote. 2019;27(6):367-374. [49] HU X, DAI Z, PAN R, et al. Long-term transplantation human menstrual blood mesenchymal stem cell loaded collagen scaffolds repair endometrium histological injury. Reprod Toxicol. 2022;109:53-60. [50] ZHANG S, ZHANG R, YIN X, et al. MenSCs Transplantation Improve the Viability of Injured Endometrial Cells Through Activating PI3K/Akt Pathway. Reprod Sci. 2023;30(11):3325-3338. [51] WANG X, BAO H, LIU X, et al. Effects of endometrial stem cell transplantation combined with estrogen in the repair of endometrial injury. Oncol Lett. 2018;16(1):1115-1122. [52] SANTAMARIA X, CABANILLAS S, CERVELLÓ I, et al. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31(5):1087-1096. [53] TAN J, LI P, WANG Q, et al. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum Reprod. 2016;31(12):2723-2729. [54] CAO Y, SUN H, ZHU H, et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther. 2018;9(1):192. [55] 黎佳敏,林姣,彭婀娜,等.去细胞羊膜载体复合自体子宫内膜干细胞治疗重度宫腔粘连1例报道[J].生殖医学杂志,2020,29(4): 541-544. [56] 吴林.冻干羊膜联合人脐带间充质干细胞治疗中重度宫腔粘连的研究[D].衡阳:南华大学,2020. [57] SINGH N, SHEKHAR B, MOHANTY S, et al. Autologous Bone Marrow-Derived Stem Cell Therapy for Asherman’s Syndrome and Endometrial Atrophy: A 5-Year Follow-up Study. J Hum Reprod Sci. 2020;13(1):31-37. [58] ZHANG Y, SHI L, LIN X, et al. Unresponsive thin endometrium caused by Asherman syndrome treated with umbilical cord mesenchymal stem cells on collagen scaffolds: a pilot study. Stem Cell Res Ther. 2021;12(1):420. [59] KACZYNSKI JB, RZEPKA JK. Endometrial regeneration in Asherman’s syndrome and endometrial atrophy using Wharton’s jelly-derived mesenchymal stem cells. Ginekol Pol. 2022;93(11):904-909. [60] WANG X, WU J, XIE Y, et al. Bone marrow mesenchymal stem cell-derived extracellular vesicles facilitate endometrial injury repair by carrying the E3 ubiquitin ligase WWP1. Biochem Cell Biol. 2022; 100(4):357-369. [61] LI J, PAN Y, YANG J, et al. Tumor necrosis factor-α-primed mesenchymal stem cell-derived exosomes promote M2 macrophage polarization via Galectin-1 and modify intrauterine adhesion on a novel murine model. Front Immunol. 2022;13:945234. [62] XIONG Z, HU Y, JIANG M, et al. Hypoxic bone marrow mesenchymal stem cell exosomes promote angiogenesis and enhance endometrial injury repair through the miR-424-5p-mediated DLL4/Notch signaling pathway. PeerJ. 2024;12:e16953. [63] CHEN Y, ZHENG S, ZHAO X, et al. Unveiling the protective effects of BMSCs/anti-miR-124-3p exosomes on LPS-induced endometrial injury. Funct Integr Genomics. 2024;24(2):32. [64] MANSOURI-KIVAJ N, NAZARI A, ESFANDIARI F, et al. Homogenous subpopulation of human mesenchymal stem cells and their extracellular vesicles restore function of endometrium in an experimental rat model of Asherman syndrome. Stem Cell Res Ther. 2023;14(1):61. [65] LIU Y, ZHANG S, XUE Z, et al. Bone mesenchymal stem cells-derived miR-223-3p-containing exosomes ameliorate lipopolysaccharide-induced acute uterine injury via interacting with endothelial progenitor cells. Bioengineered. 2021;12(2):10654-10665. [66] TAN Q, XIA D, YING X. miR-29a in Exosomes from Bone Marrow Mesenchymal Stem Cells Inhibit Fibrosis during Endometrial Repair of Intrauterine Adhesion. Int J Stem Cells. 2020;13(3):414-423. [67] XIAO B, ZHU Y, HUANG J, et al. Exosomal transfer of bone marrow mesenchymal stem cell-derived miR-340 attenuates endometrial fibrosis. Biol Open. 2019;8(5):bio039958. [68] XIONG Z, MA Y, HE J, et al. Apoptotic bodies of bone marrow mesenchymal stem cells inhibit endometrial stromal cell fibrosis by mediating the Wnt/β-catenin signaling pathway. Heliyon. 2023; 9(11):e20716. [69] XIN L, WEI C, TONG X, et al. In situ delivery of apoptotic bodies derived from mesenchymal stem cells via a hyaluronic acid hydrogel: A therapy for intrauterine adhesions. Bioact Mater. 2021;12:107-119. [70] YUAN D, GUO T, QIAN H, et al. Exosomal miR-543 derived from umbilical cord mesenchymal stem cells ameliorates endometrial fibrosis in intrauterine adhesion via downregulating N-cadherin. Placenta. 2023;131:75-81. [71] WANG S, LIU T, NAN N, et al. Exosomes from Human Umbilical Cord Mesenchymal Stem Cells Facilitates Injured Endometrial Restoring in Early Repair Period through miR-202-3p Mediating Formation of ECM. Stem Cell Rev Rep. 2023;19(6):1954-1964. [72] SHI Q, WANG D, DING X, et al. Exosome-shuttled miR-7162-3p from human umbilical cord derived mesenchymal stem cells repair endometrial stromal cell injury by restricting APOL6. Arch Biochem Biophys. 2021;707:108887. [73] SONG M, MA L, ZHU Y, et al. Umbilical cord mesenchymal stem cell-derived exosomes inhibits fibrosis in human endometrial stromal cells via miR-140-3p/FOXP1/Smad axis. Sci Rep. 2024;14(1):8321. [74] WANG J, HU R, XING Q, et al. Exosomes Derived from Umbilical Cord Mesenchymal Stem Cells Alleviate Mifepristone-Induced Human Endometrial Stromal Cell Injury. Stem Cells Int. 2020;2020:6091269. [75] LIU H, ZHANG X, ZHANG M, et al. Mesenchymal Stem Cell Derived Exosomes Repair Uterine Injury by Targeting Transforming Growth Factor-β Signaling. ACS Nano. 2024;18(4):3509-3519. [76] ZHAO S, QI W, ZHENG J, et al. Exosomes Derived from Adipose Mesenchymal Stem Cells Restore Functional Endometrium in a Rat Model of Intrauterine Adhesions. Reprod Sci. 2020;27(6):1266-1275. [77] SUN H, DONG J, FU Z, et al. TSG6-Exo@CS/GP Attenuates Endometrium Fibrosis by Inhibiting Macrophage Activation in a Murine IUA Model. Adv Mater. 2024;36(21):e2308921. [78] ZHANG S , CHANG Q , LI P , et al. Concentrated small extracellular vesicles from menstrual blood-derived stromal cells improve intrauterine adhesion, a pre-clinical study in a rat model. Nanoscale. 2021;13(15):7334-7347. [79] ZHOU L, DONG L, LI H, et al. Mesenchymal stem cell-derived exosomes ameliorate TGF-β1-induced endometrial fibrosis by altering their miRNA profile. Am J Transl Res. 2023;15(5):3203-3216. [80] 王艳阳,刘婵,余丽梅,等.间充质干细胞及细胞外囊泡治疗肺纤维化的现状与未来[J].中国组织工程研究,2024,28(25):4079-4086. [81] 熊正花,刘贝贝,杨琳娟,等.低氧处理骨髓间充质干细胞来源的外泌体对宫腔粘连模型大鼠的疗效研究[J].中华妇产科杂志, 2023,58(12):911-921. [82] LONG L, JI D, HU C, et al. Microneedles for in situ tissue regeneration. Mater Today Bio. 2023;19:100579. [83] LI X, LV HF, ZHAO R, et al. Recent developments in bio-scaffold materials as delivery strategies for therapeutics for endometrium regeneration. Mater Today Bio. 2021 ;11:100101. [84] 邬浩明,王瑶,陈圆梦,等.宫腔粘连中水凝胶促内膜修复的研究与进展[J].中国组织工程研究,2024,28(17):2774-2781. [85] LiU T, HE B, XU X. Repairing and Regenerating Injured Endometrium Methods. Reprod Sci. 2023;30(6):1724-1736. [86] ÇIL N, YAKA M, ÜNAL MS, et al. Adipose derived mesenchymal stem cell treatment in experimental asherman syndrome induced rats. Mol Biol Rep. 2020;47(6):4541-4552. [87] XIAO B, YANG W, LEI D, et al. PGS Scaffolds Promote the In Vivo Survival and Directional Differentiation of Bone Marrow Mesenchymal Stem Cells Restoring the Morphology and Function of Wounded Rat Uterus. Adv Healthc Mater. 2019;8(5):e1801455. |
| [1] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [2] | Yu Ting, Lyu Dongmei, Deng Hao, Sun Tao, Cheng Qian. Icariin pretreatment enhances effect of human periodontal stem cells on M1-type macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1328-1335. |
| [3] | Yang Zhihang, Sun Zuyan, Huang Wenliang, Wan Yu, Chen Shida, Deng Jiang. Nerve growth factor promotes chondrogenic differentiation and inhibits hypertrophic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1336-1342. |
| [4] | Hu Taotao, Liu Bing, Chen Cheng, Yin Zongyin, Kan Daohong, Ni Jie, Ye Lingxiao, Zheng Xiangbing, Yan Min, Zou Yong. Human amniotic mesenchymal stem cells overexpressing neuregulin-1 promote skin wound healing in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1343-1349. |
| [5] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [6] | Li Dijun, Jiu Jingwei, Liu Haifeng, Yan Lei, Li Songyan, Wang Bin. Three-dimensional gelatin microspheres loaded human umbilical cord mesenchymal stem cells for chronic tendinopathy repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1356-1362. |
| [7] | Lou Guo, Zhang Min, Fu Changxi. Exercise preconditioning for eight weeks enhances therapeutic effect of adipose-derived stem cells in rats with myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1363-1370. |
| [8] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [9] | Huang Ting, Zheng Xiaohan, Zhong Yuanji, Wei Yanzhao, Wei Xufang, Cao Xudong, Feng Xiaoli, Zhao Zhenqiang. Effects of macrophage migration inhibitory factor on survival, proliferation, and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1380-1387. |
| [10] | Aikepaer · Aierken, Chen Xiaotao, Wufanbieke · Baheti. Osteogenesis-induced exosomes derived from human periodontal ligament stem cells promote osteogenic differentiation of human periodontal ligament stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1388-1394. |
| [11] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [12] | Xie Liugang, Cui Shuke, Guo Nannan, Li Aoyu, Zhang Jingrui. Research hotspots and frontiers of stem cells for Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1475-1485. |
| [13] | Chang Jinxia, Liu Yufei, Niu Shaohui, Wang Chang, Cao Jianchun. Visualization analysis of macrophage polarization in tissue repair process [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1486-1496. |
| [14] | Li Jialin, Zhang Yaodong, Lou Yanru, Yu Yang, Yang Rui. Molecular mechanisms underlying role of mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1512-1522. |
| [15] | Cao Yue, Ye Xinjian, Li Biyao, Zhang Yining, Feng Jianying. Effect of extracellular vesicles for diagnosis and therapy of oral squamous cell carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1523-1530. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||