Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (31): 6800-6810.doi: 10.12307/2025.684
Previous Articles Next Articles
Mesenchymal stem cells for treatment of aplastic anemia: inhibiting or activating relevant targets in its pathological evolution
Zhang Pulian, Liu Baoru, Yang Min
- Department of Hematology, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China
-
Received:2024-07-17Accepted:2024-08-27Online:2025-11-08Published:2025-02-27 -
Contact:Yang Min, Professor, Master’s supervisor, Department of Hematology, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
About author:Zhang Pulian, Master candidate, Department of Hematology, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
Supported by:Doctoral Research Initiation Fund Project, No. [Faculty Letter (2023) 02] (to LBR); Guizhou Provincial Basic Research Program Project, No. [Qian Kehe Basic-ZK (2022) General 651] (to LBR); Project of the Department of Education of Guizhou Province, No. JZ-2021-9 (to LBR)
CLC Number:
Cite this article
Zhang Pulian, Liu Baoru, Yang Min . Mesenchymal stem cells for treatment of aplastic anemia: inhibiting or activating relevant targets in its pathological evolution[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6800-6810.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

2.2 再生障碍性贫血的发病机制 再生障碍性贫血发病机制复杂,产生的主要原因有造血干细胞缺陷(种子学说)、造血微环境异常(土壤学说)、免疫异常(虫子学说)和遗传因素[13-14]。下面主要从这4个方向阐述再生障碍性贫血发病机制的研究进展,希望为治疗再生障碍性贫血提供新的策略和理论基础。 2.2.1 造血干细胞缺陷(种子学说) 造血干细胞在造血微环境的调节下分化为各种血细胞和祖细胞,从而维持正常的骨髓造血。病毒、化疗药物或辐射等因素直接影响造血干细胞的自我更新和分化功能,导致全血细胞减少、造血功能受损[15-16]。再生障碍性贫血患者T细胞异常活化后产生过多的干扰素γ,干扰素γ诱导细胞因子信号传导抑制蛋白(suppressor of cytokine signaling,SOCS)的表达,如SOCS1和SOCS3,进而抑制信号传导及转录激活蛋白(signal transducer and activator of transcription,STAT)的传导,干扰血小板生成素和粒细胞集落刺激因子的效应,造成造血干细胞数量减少[17]。也有研究指出干扰素γ可能通过阻碍血小板生成素与血小板生成素受体之间的相互作用来发挥对造血的抑制作用,使用血小板生成素受体激动剂艾曲波帕后改善了骨髓造血也证明了这一点[18-19]。细胞死亡受体(Fas)及其配体(FasL)形成的Fas/FasL凋亡通路与多种疾病的发病机制有关。当体内产生过多的炎性细胞因子,使得CD34细胞上的Fas表达水平上升,激活Fas/FasL凋亡通路,诱导造血干细胞的凋亡 [20]。HUANG等[21]研究了自噬对健康成人和再生障碍性贫血患者骨髓CD34+细胞存活的影响,发现再生障碍性贫血患者CD34细胞的自噬相关标志物LC3-Ⅱ水平显著降低,并且重度再生障碍性贫血组低于非重度再生障碍性贫血组,结果表明再生障碍性贫血患者CD34细胞自噬受损。CD34细胞的自噬受损可能在再生障碍性贫血的发病机制中发挥了重要作用。 2.2.2 造血微环境异常(土壤学说) 骨髓造血微环境主要是由造血干细胞生态位,包括基质细胞、血管周围细胞和骨内膜细胞组成[22]。间充质干细胞是造血微环境的核心组成细胞,间充质干细胞功能异常时,产生的干扰素γ、白细胞介素2、白细胞介素17、肿瘤坏死因子α和穿孔素水平显著升高,支持造血的细胞因子减少,如干细胞因子、血管内皮生长因子、转化生长因子β和血管生成素1,激活Fas凋亡通路,骨髓内环境免疫稳态失调,使得骨髓组织和造血细胞减少 [16,23]。功能失调后的骨髓间充质干细胞成骨分化能力下降,脂肪分化能力显著提高,驱动骨髓组织向脂肪化发展,见图4。LU等[24]将再生障碍性贫血患者的间充质干细胞与健康供者相比,发现再生障碍性贫血患者间充质干细胞表达的血管细胞黏附分子1(CD106)显著低于健康供者,导致内皮细胞分化和血管形成能力下降。有研究将再生障碍性贫血小鼠的内皮细胞功能使用拮抗剂(N-乙酰基-L-半胱氨酸)阻断后,进行体外内皮细胞输注,发现细胞输注后缓解了造血受损[25-26],表明再生障碍性贫血患者的造血能力下降与内皮损伤有关,对内皮细胞进行功能修复可能是一种有效的治疗再生障碍性贫血的方法。SHIPOUNOVA等[27]将再生障碍性贫血患者的骨髓样本进行体外培养,RT-PCR检测到血管生成素1和血管细胞黏附分子1表达水平下降,成骨细胞和血管的异常调节可能进一步加重了造血损伤。 "


2.2.3 免疫异常(虫子学说) 免疫系统的异常激活是目前再生障碍性贫血较公认的发病机制,主要是由于造血微环境中失调的免疫细胞和免疫相关因子相互作用共同导致造血干细胞功能缺陷。这些免疫细胞主要包括T淋巴细胞(CD4 T、CD8 T)、自然杀伤细胞、树突细胞 [28]。 (1)T细胞:在再生障碍性贫血患者中,患者骨髓和外周血中活化的细胞毒性T淋巴细胞的百分比增加,活化的细胞毒性T淋巴细胞被认为是抑制再生障碍性贫血患者造血的最重要效应T细胞。CD8+ T细胞数目显著增加,CD8+/CD4+ T细胞比例倒置,一方面,CD8+ T细胞分泌的干扰素γ、白细胞介素2、肿瘤坏死因子β和穿孔素等Ⅰ型淋巴因子表现出增强的功能,这些细胞因子可促进重度再生障碍性贫血患者细胞毒性T淋巴细胞激活,进而抑制骨髓造血[29];另一方面,CD8+ T效应细胞可通过肿瘤坏死因子β相关通路、颗粒酶B途径、Fas/FasL凋亡通路损伤患者的骨髓造血细胞[30],再生障碍性贫血的严重程度和免疫异常与Th17/Tregs平衡失调有关,再生障碍性贫血患者CD4+ T细胞分化的Th1、Th17细胞增加,Tregs减少,Tregs的缺陷进一步刺激T细胞扩增,使得Th1/Th2、Th17/Tregs比例失衡,进而导致大量的Ⅰ型淋巴因子产生和免疫失调[31-34]。 (2)树突细胞:树突细胞是激活幼稚T细胞重要的抗原递呈细胞,髓系树突状细胞在T细胞介导的免疫应答中起着独特的作用[35]。髓系树突状细胞可促进Th0向Th1极化,进而启动细胞免疫。再生障碍性贫血患者的髓系树突状细胞处于过度激活状态,与Th1细胞相关的细胞因子(干扰素γ、肿瘤坏死因子α、白细胞介素2)显著增加,导致髓系树突状细胞抗原呈递增强,细胞毒性T淋巴细胞过度活化,进而破坏骨髓中的造血细胞[36]。有研究对重度再生障碍性贫血患者和健康人的髓系树突状细胞吞噬功能进行了比较,结果表明重度再生障碍性贫血患者髓系树突状细胞的吞噬指数相比健康人显著提高,并与外周血水平呈负相关[37],表明肌动蛋白调节因子Cofilin-1可能是再生障碍性贫血进展和治疗的潜在指标,髓系树突状细胞吞噬功能的增强参与了再生障碍性贫血的发病机制。SUN等[38]对重度再生障碍性贫血患者髓系树突状细胞的cofilin-1进行敲低后,显著降低了重度再生障碍性贫血患者髓系树突状细胞的吞噬作用以及CD86的表达。下调髓系树突状细胞中Cofilin-1的表达可能是治疗再生障碍性贫血的潜在靶点。 (3)自然杀伤细胞:自然杀伤细胞是参与先天免疫的一个重要免疫细胞,在免疫调节、免疫监视、抗肿瘤等方面起着重要作用。自然杀伤细胞根据表面标记物CD16和CD56的相对表达细分为CD16(-)、CD56(+)和CD16(+)、CD56(-)。有研究证明再生障碍性贫血患者的外周血自然杀伤细胞CD56(+)、CD56(-)百分比降低,穿孔素表达较高,并且CD56表达百分比与骨髓增生程度呈正相关[39],表明自然杀伤细胞参与形成了再生障碍性贫血的造血功能低下,但作用机制未详细说明,需要做进一步研究。 2.2.4 遗传因素 遗传因素(如端粒酶异常、体细胞突变和遗传易感性)在再生障碍性贫血的发病机制中起着重要作用[40]。端粒是保护性染色体帽,在细胞复制期间或暴露于应激源后会缩短。MELGUIZO-SANCHIS等[41]建立了模拟再生障碍性贫血的体外细胞模型,与健康对照组相比,再生障碍性贫血患者的端粒长度较短,端粒酶相关基因(如TERC、TERT和DKC)存在突变,影响了造血干细胞的多能表型和分化能力,表明端粒酶活性可能与再生障碍性贫血的发病或病理进展有关。YOSHIZATO等[42]利用439例再生障碍性贫血患者的668份血液样本进行了新一代测序和基于阵列的核型分析,报告显示47%的患者存在干细胞克隆性造血,伴有体细胞突变。克隆进化是再生障碍性贫血患者病情演变的严重不良事件,即骨髓增生异常综合征和急性髓系白血病的发展[43]。再生障碍性贫血中最常见的突变基因包括PIGA、BCOR、BCORL1,这些基因突变是相对良性/轻度的突变,可预测免疫抑制治疗的疗效和预测不利的结果,从而改善总生存期。基因突变率、克隆性造血与向骨髓增生异常综合征/急性髓系白血病/阵发性睡眠性血红蛋白尿症的转化和发病风险有关[44]。治疗过程中进行基因组检测分析可能有助于对突变风险高的患者进行适当和适时的管理。 2.3 间充质干细胞 2.3.1 间充质干细胞的生物特性 间充质干细胞是一种多能干细胞,具有调节免疫反应、支持造血、促进组织再生等功能。间充质干细胞来源广泛,可从骨髓、脐血、胎盘等组织中获取。国际细胞治疗学会(ISCT)定义了间充质干细胞的基本标准[45]:①贴壁生长;②体外培养可分化为成骨细胞、脂肪细胞和软骨细胞;③表面抗原CD105、CD73和CD90高表达,表面抗原CD45、CD34、CD14或CD11b、CD79a或CD19、HLA-DR低表达或不表达,见图5。间充质干细胞体外培养可分化为多种类型的细胞,如心肌细胞、肝细胞、神经细胞[46-48]。间充质干细胞来源广泛、不存在伦理问题、易制备等特点使其成为在细胞疗法中优先的选择。 "


2.3.2 间充质干细胞的免疫特性 普遍认为,间充质干细胞主要是通过旁分泌效应来行使免疫调节、抗凋亡、抑制炎症等功能。间充质干细胞依赖于趋化因子发挥免疫作用,其免疫抑制活性主要受促炎因子刺激而引发,如肿瘤坏死因子α、干扰素γ、白细胞介素1β [49]。再生障碍性贫血患者体内产生大量的促炎因子激活了间充质干细胞的免疫抑制性,进一步抑制T淋巴细胞、B淋巴细胞、树突细胞和自然杀伤细胞的活化与增殖,抑制机体免疫反应,从而延缓疾病的病理演变过程[49]。Tregs对于免疫稳态以及预防同种异体反应和自身免疫性疾病至关重要,间充质干细胞可抑制干扰素γ和白细胞介素17分泌,并抑制CD4 T细胞向Th1和 Th17细胞分化,促进CD4 T细胞向Tregs分化。此外,间充质干细胞分泌白细胞介素6、白细胞介素8、白细胞介素10、转化生长因子β等免疫抑制因子,抑制Th1和Th17细胞的激活,调节Tregs的功能[50]。在炎症环境下,间充质干细胞募集巨噬细胞到炎症部位作用于炎症因子,并通过吲哚胺1,2-双加氧酶、C-C基序配体3和前列腺素E18,诱导巨噬细胞从促炎性M1型向抗炎性M2型转变,进而发挥抗炎及免疫抑制效应[51]。间充质干细胞可抑制Th1促炎细胞因子的表达并增强Th2因子的表达,从而产生更耐受的免疫状态。间充质干细胞不表达MHCⅡ、HLA-DR、B7-1、CD40及Fas 配体,因此很难被机体的免疫系统识别,减少了外源性抗原在体内产生免疫反应的机会。体内免疫失调产生过多的Th1、 Th17和干扰素γ是导致再生障碍性贫血和移植物抗宿主病发生的关键过程,因此,输注间充质干细胞对再生障碍性贫血患者治疗,一方面减少了体内免疫排斥的发生,另一方面免疫抑制功能可对再生障碍性贫血和移植物抗宿主病进行预防和治疗。近年来,间充质干细胞独特低免疫原性已被用于多发性硬化症、克罗恩病、系统性红斑狼疮等免疫相关疾病的治疗[52-54]。 2.3.3 间充质干细胞维持正常的造血功能 在造血微环境中,骨髓间充质干细胞占领基质细胞的核心地位。骨髓间充质干细胞分泌多种造血相关因子和免疫调节因子调节造血干细胞的动员、迁移、增殖和分化。间充质干细胞低(或不)表达造血干细胞相关表面抗原,产生多种细胞因子和可溶性物质支持着造血干细胞的生长和发育,如血小板生成素、白细胞介素6、粒细胞集落刺激因子、干细胞因子、Flt-3配体支持造血干细胞的增殖和分化,产生的趋化因子12 (也称为基质细胞衍生因子1)、血管细胞黏附分子1、血管内皮生长因子在造血干细胞的动员、归巢和迁移中有重要作用[55]。间充质干细胞的旁分泌物参与信号通路的传导,有一些信号通路已被证明与造血有关,这些信号通路的受体包括丝氨酸/苏氨酸激酶、信号传导及转录激活蛋白、酪氨酸激酶受体2/血管生成素1、p38丝裂原活化蛋白激酶和肿瘤坏死因子。间充质干细胞上表达的基质细胞衍生因子1可选择性激活信号传导及转录激活蛋白5,也可激活丝裂原活化蛋白激酶信号通路,这两种信号通路分别诱导造血干细胞增殖和分化[55]。基质细胞衍生因子1与造血干细胞上的CXCR4受体结合形成CXCL12/CXCR4轴参与了造血干细胞的动员和归巢。此外,CXCL12/CXCR4轴可激活多种信号通路,如Janus激酶/ 信号传导及转录激活蛋白3通路、GTP 结合蛋白通路、p38丝裂原活化蛋白激酶通路、Notch 配体1/神经源性基因Notch同源蛋白1/Hes家族转录因子通路、应激活化蛋白激酶/Jun 氨基末端激酶通路,这些通路的传导以及下游分子的激活参与了造血和细胞凋亡的调节、癌细胞的生长迁移和新生血管形成[56-57]。选择性抑制造血干细胞凋亡或激活造血相关通路,可能是缓解再生障碍性贫血患者造血干细胞数量急剧下降的重要手段。间充质干细胞释放的胞外囊泡有着与间充质干细胞相似的生物特性与免疫特点,是细胞微环境中重要的细胞间信息交流的通讯递质。尽管细胞外囊泡的生理功能尚未完全了解,但有研究表明,间充质干细胞外囊泡可以重塑造血功能,通过将其含有的蛋白质、脂质和核酸等物质转移到造血干细胞,从而影响造血干细胞的生成[58]。间充质干细胞通过细胞接触、分泌可溶性因子、参与多条通路支持造血功能,这也为间充质干细胞应用在造血恢复中提供了理论基础。 2.4 间充质干细胞在再生障碍性贫血中的致病机制 间充质干细胞及其衍生物已被证明参与了再生障碍性贫血的形成过程,在再生障碍性贫血中也同样证实了骨髓间充质干细胞存在缺陷。与健康者间充质干细胞相比,再生障碍性贫血患者间充质干细胞在增殖、成骨分化、免疫调节和造血支持方面都表现出与再生障碍性贫血一致的差异[59]。一项关于再生障碍性贫血患者骨髓间充质干细胞的研究表明,血管内皮生长因子-Notch 信号传导受到显著抑制,间充质干细胞增殖减少,凋亡增加,可通过体外激活血管内皮生长因子-Notch信号恢复再生障碍性贫血患者间充质干细胞的增殖功能[60]。再生障碍性贫血患者的间充质干细胞成骨潜力降低,而成脂能力增加,是导致骨髓脂肪化的主要原因。miRNA是一类小的(21-23 nt)内源性非编码RNA,可通过miRNA种子序列与靶向RNA的碱基配对来调控基因表达,ZHANG等[61]发现再生障碍性贫血骨髓间充质干细胞中miR-199a-5p的表达量显著增加,其过表达可增强间充质干细胞的成脂分化倾向, miR-199a-5p通过诱导靶向转化生长因子β的表达来调节成脂分化,转化生长因子β是脂肪生成的负调节因子,miR-199a-5p与转化生长因子β的表达呈负相关。在再生障碍性贫血患者中,miR-199a-5p/转化因子通路参与成骨/成脂失衡,导致骨髓脂肪细胞大量增多,通过抑制miR-199a-5p或增强转化生长因子β表达可能是治疗再生障碍性贫血的一个潜在靶点。间充质干细胞的免疫调节缺陷在再生障碍性贫血的病理形成机制中扮演着重要的角色,为了评估重度再生障碍性贫血患者间充质干细胞对T细胞分化的影响,有研究将Tregs和Th17细胞极化后的小鼠CD4+ T 细胞与间充质干细胞共培养,结果显示重度再生障碍性贫血患者间充质干细胞抑制Th17激活的能力受损和产生大量功能失调Tregs,而且白细胞介素1β、干扰素γ和肿瘤坏死因子α水平较高[62],这意味着再生障碍性贫血患者间充质干细胞的免疫调节功能紊乱的同时也可能出现与免疫紊乱相关的高炎症环境。Li等[63]对15例再生障碍性贫血患者静脉注射骨髓间充质干细胞,结果显示Th1和Th17细胞显著降低,炎症因子显著下降,也证实了上述结论。 在遗传学方面,基因芯片检测显示,与健康对照组相比,再生障碍性贫血患者骨髓间充质干细胞参与细胞周期、细胞增殖、分化、造血和免疫应答相关的基因表达显著下降,但与细胞凋亡、脂肪生成和免疫反应相关的基因表达增加[64]。在再生障碍性贫血的病理演变过程中,间充质干细胞进一步抑制了造血干细胞增殖,驱动骨髓脂肪化状态形成。HUO等[65]系统评估了39名健康供者和64例再生障碍性贫血患者的骨髓间充质干细胞之间的差异,发现再生障碍性贫血患者间充质干细胞的形态与健康供者间充质干细胞不同(大而肿胀,而非纺锤形),而免疫表型则相当;定量RT-PCR分析显示,再生障碍性贫血患者间充质干细胞的成脂潜能增加(脂联素和过氧化物酶体增殖物激活受体γ水平升高),而成骨潜能降低(Runt 相关转录因子2和骨γ-羧基谷氨酸蛋白水平降低);此外,全基因组RNA测序显示共有19 138个差异表达基因,再生障碍性贫血中上调的基因涉及免疫反应(即肿瘤坏死因子、Toll样受体、白细胞介素17)、细胞分裂、细胞黏附(细胞黏附分子)、细胞周期和分化;最后,剪接体分析表明,再生障碍性贫血患者间充质干细胞与健康供者间充质干细胞在组蛋白去乙酰化酶活性、细胞生长和各种通路(即Wnt、雷帕霉素靶蛋白、Hippo、Notch和血管内皮生长因子)方面存在不同的剪接体。这项非常全面的研究为骨髓微环境和间充质干细胞参与再生障碍性贫血的形成机制提供了重要的依据。 表1总结了再生障碍性贫血中间充质干细胞的特性。"

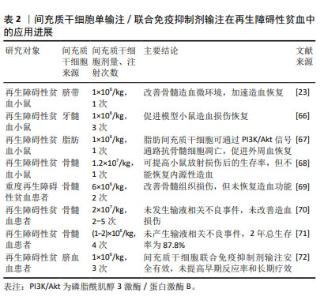
2.5 间充质干细胞在再生障碍性贫血中的治疗应用 2.5.1 间充质干细胞输注治疗再生障碍性贫血 为探究间充质干细胞单输注对再生障碍性贫血的疗效,多项研究将人间充质干细胞输注于再生障碍性贫血小鼠模型,在这些研究中,间充质干细胞的来源很多,包括骨髓、脐带、脂肪组织和牙龈组织,均证实了间充质干细胞可提高小鼠的存活率、恢复造血损伤[23,65-67]。然而,DIAZ等[68]将骨髓间充质干细胞注射于电离辐射的急性造血衰竭(H-ARS)小鼠体内,显示只对放射敏感组织(如肠黏膜)产生积极影响,并未恢复内源性造血功能。由此可见,在再生障碍性贫血小鼠体内,间充质干细胞的疗效是存在争议的。在再生障碍性贫血患者体内,有2项静脉多次输注骨髓间充质干细胞治疗难治性或复发性再生障碍性贫血患者的研究中,也同样只证实了骨髓间充质干细胞的安全性,并未重建或恢复再生障碍性贫血患者的造血功能[69-70]。 一项多中心Ⅱ期研究中,接受同种异体骨髓间充质干细胞输注的74例难治性再生障碍性贫血患者,1年客观缓解率为28.4%,2年总生存率为87.8%,未产生输液相关不良事件[71]。另外一项慢性再生障碍性贫血患者输注骨髓间充质干细胞的研究中,骨髓间充质干细胞通过影响 Notch/RBP-J/FOXP3/RORγt 通路调节了Tregs/Th17的平衡,Tregs百分比增加[63]。间充质干细胞输注对难治性或复发性再生障碍性贫血的疗效也同样存在争议,但大部分研究表明无论是单次还是多次输注间充质干细胞,都不能重建再生障碍性贫血的造血功能。研究结果存在争议可能与样本量、样本异质性、间充质干细胞来源差异等因素有关,还需要大样本的对照研究来明确间充质干细胞输注对再生障碍性贫血的疗效。 2.5.2 间充质干细胞联合免疫抑制剂治疗再生障碍性贫血 关于免疫抑制剂联合间充质干细胞输注治疗再生障碍性贫血的研究较少,多为免疫抑制剂治疗再生障碍性贫血无效形成的难治性或复发性再生障碍性贫血,再进行间充质干细胞输注疗效探究的研究类型。LAN等[72]进行的一项前瞻性Ⅳ期试验中,比较了免疫抑制剂单独治疗和联合骨髓间充质干细胞输注治疗重度再生障碍性贫血患儿之间的疗效差异,结果表明与免疫抑制剂单独治疗相比,骨髓间充质干细胞联合免疫抑制剂治疗并未提高重度再生障碍性贫血患者的早期反应率,改善患者的远期疗效。该研究样本量小和入组患者存在明显异质性,一些结果可能不具有代表性,还需要进行样本量更大的前瞻性研究。 表2总结了间充质干细胞单输注/联合免疫抑制剂输注在再生障碍性贫血中的应用进展。 "

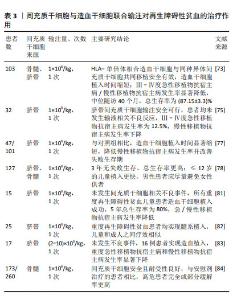
2.5.3 间充质干细胞与造血干细胞联合移植治疗再生障碍性贫血的进展 鉴于间充质干细胞单纯输注、联合免疫抑制剂输注对再生障碍性贫血患者疗效不理想的现状,联合造血干细胞输注对于再生障碍性贫血患者可能是更好的选择。临床上关于间充质干细胞联合造血干细胞输注治疗再生障碍性贫血的疗效也有目共睹。 (1)增加造血干细胞植入成功率:中国独生子女较多,全相合供者少,造成造血干细胞植入成功率下降。多项研究表明,间充质干细胞联合造血干细胞输注可以增强造血干细胞的植入成功率进而减少移植失败的发生[73-76]。SHENG等[77]进行的一项回顾性对照研究中,比较了接受单倍体相合造血干细胞移植(haplo-HSCT)联合或不联合脐带间充质干细胞治疗重度再生障碍性贫血患者的疗效,脐带间充质干细胞参与治疗的患者植入时间显著缩短,5年无移植物抗宿主病、无失败生存期显著改善,表明间充质干细胞联合造血干细胞移植治疗再生障碍性贫血不仅可以缩短植入时间,促进造血干细胞的植入,还可提高患者的生活质量,改善患者的生存结果。韩冬梅等[78]的一项回顾性研究中,127例接受造血干细胞联合脐带间充质干细胞移植治疗的重度再生障碍性贫血患者,造血干细胞植入成功率为99.2%,3年总生存率为86.1%,Ⅲ-Ⅳ度急性移植物抗宿主病发生率明显降低,3年无失败生存更高,并且单因素分析结果显示,儿童患者预后优于成人,供者为女性的男性患者慢性移植物抗宿主病累积发生率更高。重度再生障碍性贫血患者应尽早实施造血干细胞移植,使用男性供者的造血干细胞移植给男性再生障碍性贫血患者可能更有利于恢复。虽然这些研究结论都证明了间充质干细胞的有效性,但这些研究中样本量都较少,联合移植的疗效差异可能与个体、患者年龄、间充质干细胞数量等因素有关,还需要更大样本量的临床试验研究制定标准化治疗方案。 (2)预防和治疗移植物抗宿主病:移植物抗宿主病是再生障碍性贫血患者进行异基因造血干细胞移植后最常见的并发症,也是造成移植失败的主要原因。移植物抗宿主病主要是由供者T淋巴细胞攻击受者组织引起,受者细胞因子刺激,然后攻击受者体内的同种异体抗原。按起病时间分为急性移植物抗宿主病和慢性移植物抗宿主病 [79]。供者T细胞活化后刺激其他效应细胞的募集是导致移植物抗宿主病的重要原因,这些效应细胞主要是T细胞、树突细胞、B细胞和自然杀伤细胞[80]。间充质干细胞作为低免疫原性细胞,用于预防和治疗移植物抗宿主病有着良好的前景。有研究表明再生障碍性贫血患者在造血干细胞移植期间输注间充质干细胞联合移植可以增强造血重建、防止移植物排斥、改善移植物抗宿主病[81-82]。LI等[83]研究了17例造血干细胞移植联合间充质干细胞输注治疗重度再生障碍性贫血患者是否可以预防移植失败和移植物抗宿主病,结果显示,轻度急性移植物抗宿主病发生率无明显变化,Ⅲ-Ⅳ级急性移植物抗宿主病的发生率为23.5%,中重度慢性移植物抗宿主病的发生率为14.2%,有效降低了重度移植物抗宿主病的发生率。皮质类固醇是急性移植物抗宿主病的一线治疗药物,总体缓解率为67%-80%,激素耐药的急性移植物抗宿主病患者总生存率低于10%[10]。在一项关于评估间充质干细胞与安慰剂在难治性急性移植物抗宿主病患者疗效的Ⅲ期随机研究中,与接受安慰剂治疗的患者相比,接受间充质干细胞输注治疗的患者有更高的持久完全缓解率和总体完全或部分缓解率[84]。这些研究表明在造血干细胞移植期间,间充质干细胞联合移植可以降低重度移植物抗宿主病的发生率,对于难治性移植物抗宿主病患者而言,间充质干细胞的使用可以提高患者的生存率,也能减少移植失败和移植物抗宿主病的发生。然而,这些研究的样本量太小,还需要大量的临床试验进行进一步的前瞻性研究来验证这些结论。 表3总结了间充质干细胞与造血干细胞联合输注对再生障碍性贫血的治疗作用。 "

| [1] DEZERN AE, CHURPEK JE. Approach to the diagnosis of aplastic anemia. Blood Adv. 2021;5(12):2660-2671. [2] AHMED P, CHAUDHRY QUN, SATTI TM, et al. Epidemiology of aplastic anemia: a study of 1324 cases. Hematology. 2020;25(1):48-54. [3] PATEL BA, TOWNSLEY DM, SCHEINBERG P. Immunosuppressive therapy in severe aplastic anemia. Semin Hematol. 2022;59(1):21-29. [4] PAN P, CHEN C, HONG J, et al. Autoimmune pathogenesis, immunosuppressive therapy and pharmacological mechanism in aplastic anemia. Int Immunopharmacol. 2023;117:110036. [5] WILFRED G, ONG TC, SH SHAHNAZ SAK, et al. Allogeneic Hematopoietic Stem Cell Transplantation in Severe Aplastic Anemia: A Single Centre Experience in Malaysia. Blood Cell Ther. 2022;5(2):45-53. [6] ZIELIŃSKA P, NOSTER I, WIECZORKIEWICZ-KABUT A, et al. Allogeneic hematopoietic stem cell transplantation for acquired severe aplastic anemia: a summary of a 20-year experience. Pol Arch Intern Med. 2023;133(7-8):16448. [7] VAN LIER YF, VOS J, BLOM B, et al. Allogeneic hematopoietic cell transplantation, the microbiome, and graft-versus-host disease. Gut Microbes. 2023;15(1):2178805. [8] WESTIN JR, SALIBA RM, DE LIMA M, et al. Steroid-Refractory Acute GVHD: Predictors and Outcomes. Adv Hematol. 2011;2011:601953. [9] GARRIGÓS MM, DE OLIVEIRA FA, NUCCI MP, et al. How mesenchymal stem cell cotransplantation with hematopoietic stem cells can improve engraftment in animal models. World J Stem Cells. 2022;14(8):658-679. [10] LIN T, YANG Y, CHEN X. A review of the application of mesenchymal stem cells in the field of hematopoietic stem cell transplantation. Eur J Med Res. 2023;28(1):268. [11] WANG E, ZHANG Y, DING R, et al. miR‑30a‑5p induces the adipogenic differentiation of bone marrow mesenchymal stem cells by targeting FAM13A/Wnt/β‑catenin signaling in aplastic anemia. Mol Med Rep. 2022;25(1):27. [12] DENG S, ZENG Y, XIANG J, et al. Icariin protects bone marrow mesenchymal stem cells in aplastic anemia by targeting MAPK pathway. Mol Biol Rep. 2022;49(9):8317-8324. [13] GIUDICE V, SELLERI C. Aplastic anemia: Pathophysiology. Semin Hematol. 2022;59(1):13-20. [14] DURRANI J, GROARKE EM. Clonality in immune aplastic anemia: Mechanisms of immune escape or malignant transformation. Semin Hematol. 2022;59(3):137-142. [15] GONNOT M, NEUMANN F, HUET F, et al. Hepatitis-associated Aplastic Anemia. J Pediatr Gastroenterol Nutr. 2022;75(5):553-555. [16] NI R, FAN L, ZHANG L, et al. A mouse model of irradiation and spleen-thymus lymphocyte infusion induced aplastic anemia. Hematology. 2022;27(1):932-945. [17] MERLI P, QUINTARELLI C, STROCCHIO L, et al. The role of interferon-gamma and its signaling pathway in pediatric hematological disorders. Pediatr Blood Cancer. 2021;68(4):e28900. [18] PATEL BA, GROARKE EM, LOTTER J, et al. Long-term outcomes in patients with severe aplastic anemia treated with immunosuppression and eltrombopag: a phase 2 study. Blood. 2022;139(1):34-43. [19] ALVARADO LJ, HUNTSMAN HD, CHENG H, et al. Eltrombopag maintains human hematopoietic stem and progenitor cells under inflammatory conditions mediated by IFN-γ. Blood. 2019;133(19):2043-2055. [20] ALOBAIDI A, ALBADRY A, MURRAY A. Very Severe Aplastic Anemia in a 26-Year-Old Male: Implications for Prognosis and Treatment Options. Cureus. 2023;15(9):e45750. [21] HUANG J, GE M, LU S, et al. Impaired Autophagy in Adult Bone Marrow CD34+ Cells of Patients with Aplastic Anemia: Possible Pathogenic Significance. PLoS One. 2016;11(3):e0149586. [22] WANG H, LENG Y, GONG Y. Bone Marrow Fat and Hematopoiesis. Front Endocrinol (Lausanne). 2018;9:694. [23] HE C, YANG C, ZENG Q, et al. Umbilical cord-derived mesenchymal stem cells cultured in the MCL medium for aplastic anemia therapy. Stem Cell Res Ther. 2023;14(1):224. [24] LU SH, GE ML, ZHENG YZ, et al. Effect of CD106+ Mesenchymal Stem Cell on Bone Marrow Vascular Failure in Patients with Aplastic Anemia. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2018;40(2):178-186. [25] TANG SQ, XING T, LYU ZS, et al. Repair of dysfunctional bone marrow endothelial cells alleviates aplastic anemia. Sci China Life Sci. 2023; 66(11):2553-2570. [26] TRIPATHY NK, SINGH SP, NITYANAND S. Enhanced adipogenicity of bone marrow mesenchymal stem cells in aplastic anemia. Stem Cells Int. 2014;2014:276862. [27] SHIPOUNOVA IN, PETROVA TV, SVINAREVA DA, et al. Alterations in hematopoietic microenvironment in patients with aplastic anemia. Clin Transl Sci. 2009;2(1):67-74. [28] YU W, WANG Q, GE M, et al. Cluster analysis of lymphocyte subset from peripheral blood in newly diagnosed idiopathic aplastic anaemia patients. Ann Med. 2022;54(1):2431-2439. [29] QI W, ZHANG Y, WANG Y, et al. Abnormal expression of histone acetylases in CD8+ T cells of patients with severe aplastic anemia. J Clin Lab Anal. 2022;36(4):e24339. [30] XING L, LIU C, FU R, et al. CD8+HLA-DR+ T cells are increased in patients with severe aplastic anemia. Mol Med Rep. 2014;10(3):1252-1258. [31] WEN S, HE L, ZHONG Z, et al. Stigmasterol Restores the Balance of Treg/Th17 Cells by Activating the Butyrate-PPARγ Axis in Colitis. Front Immunol. 2021;12:741934. [32] WANG J, ZHAO X, WAN YY. Intricacies of TGF-β signaling in Treg and Th17 cell biology. Cell Mol Immunol. 2023;20(9):1002-1022. [33] ZHAO SS, ZHU XJ, WU RH. Relationship Between the Changes of Regulatory T Cells and Th17 Cells and the Prognosis of Children with Aplastic Anemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2020;28(5): 1674-1678. [34] HUANG J, PU Y, XU K, et al. High expression of HIF-1α alleviates benzene-induced hematopoietic toxicity and immunosuppression in mice. Environ Pollut. 2022;311:119928. [35] LIN Q, LIU Z, LUO M, et al. Visualizing DC morphology and T cell motility to characterize DC-T cell encounters in mouse lymph nodes under mTOR inhibition. Sci China Life Sci. 2019;62(9):1168-1177. [36] LIU C, SHENG W, FU R, et al. Differential expression of the proteome of myeloid dendritic cells in severe aplastic anemia. Cell Immunol. 2013;285(1-2):141-148. [37] SUN Y, WU C, LIU C, et al. Myeloid dendritic cells in severe aplastic anemia patients exhibit stronger phagocytosis. J Clin Lab Anal. 2021; 35(12):e24063. [38] SUN Y, ZHANG Y, YU H, et al. Cofilin-1 participates in the hyperfunction of myeloid dendritic cells in patients with severe aplastic anaemia. J Cell Mol Med. 2022;26(12):3460-3470. [39] LI ZS, SHAO ZH, FU R, et al. Percentages and functions of natural killer cell subsets in peripheral blood of patients with severe aplastic anemia. Zhonghua Yi Xue Za Zhi. 2011;91(16):1084-1087. [40] BRZEŹNIAKIEWICZ-JANUS K, RUPA-MATYSEK J, GIL L. Acquired Aplastic Anemia as a Clonal Disorder of Hematopoietic Stem Cells. Stem Cell Rev Rep. 2020;16(3):472-481. [41] MELGUIZO-SANCHIS D, XU Y, TAHEEM D, et al. iPSC modeling of severe aplastic anemia reveals impaired differentiation and telomere shortening in blood progenitors. Cell Death Dis. 2018;9(2):128. [42] YOSHIZATO T, DUMITRIU B, HOSOKAWA K, et al. Somatic Mutations and Clonal Hematopoiesis in Aplastic Anemia. N Engl J Med. 2015; 373(1):35-47. [43] BABUSHOK DV, PERDIGONES N, PERIN JC, et al. Emergence of clonal hematopoiesis in the majority of patients with acquired aplastic anemia. Cancer Genet. 2015;208(4):115-128. [44] SCHOETTLER ML, NATHAN DG. The Pathophysiology of Acquired Aplastic Anemia: Current Concepts Revisited. Hematol Oncol Clin North Am. 2018;32(4):581-594. [45] DOMINICI M, LE BLANC K, MUELLER I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. [46] AFSHARI A, SHAMDANI S, UZAN G, et al. Different approaches for transformation of mesenchymal stem cells into hepatocyte-like cells. Stem Cell Res Ther. 2020;11(1):54. [47] VAN RENSBURG MJ, CROUS A, ABRAHAMSE H. Potential of Photobiomodulation to Induce Differentiation of Adipose- Derived Mesenchymal Stem Cells into Neural Cells. Curr Stem Cell Res Ther. 2021;16(3):307-322. [48] SUN Y, LIU J, XU Z, et al. Matrix stiffness regulates myocardial differentiation of human umbilical cord mesenchymal stem cells. Aging (Albany NY). 2020;13(2):2231-2250. [49] JIANG W, XU J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53(1):e12712. [50] ZHOU Y, YAMAMOTO Y, XIAO Z, et al. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J Clin Med. 2019;8(7):1025. [51] ARABPOUR M, SAGHAZADEH A, REZAEI N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int Immunopharmacol. 2021;97:107823. [52] FATHOLLAHI A, GABALOU NB, ASLANI S. Mesenchymal stem cell transplantation in systemic lupus erythematous, a mesenchymal stem cell disorder. Lupus. 2018;27(7):1053-1064. [53] WŁODARCZYK M, CZERWIŃSKA K, WŁODARCZYK J, et al. Current Overview on the Use of Mesenchymal Stem Cells for Perianal Fistula Treatment in Patients with Crohn’s Disease. Life (Basel). 2021;11(11): 1133. [54] ZHANG Y, GU J, WANG X, et al. Opportunities and challenges: mesenchymal stem cells in the treatment of multiple sclerosis. Int J Neurosci. 2023;133(9):1031-1044. [55] AQMASHEH S, SHAMSASANJAN K, AKBARZADEHLALEH P, et al. Effects of Mesenchymal Stem Cell Derivatives on Hematopoiesis and Hematopoietic Stem Cells. Adv Pharm Bull. 2017;7(2):165-177. [56] WANG H, BI X, ZHANG R, et al. Adipose-Derived Mesenchymal Stem Cell Facilitate Hematopoietic Stem Cell Proliferation via the Jagged-1/Notch-1/Hes Signaling Pathway. Stem Cells Int. 2023;2023:1068405. [57] KHARE T, BISSONNETTE M, KHARE S. CXCL12-CXCR4/CXCR7 Axis in Colorectal Cancer: Therapeutic Target in Preclinical and Clinical Studies. Int J Mol Sci. 2021;22(14):7371. [58] BUDGUDE P, KALE V, VAIDYA A. Mesenchymal stromal cell-derived extracellular vesicles as cell-free biologics for the ex vivo expansion of hematopoietic stem cells. Cell Biol Int. 2020;44(5):1078-1102. [59] ATMAR K, TULLING AJ, LANKESTER AC, et al. Functional and Immune Modulatory Characteristics of Bone Marrow Mesenchymal Stromal Cells in Patients With Aplastic Anemia: A Systematic Review. Front Immunol. 2022;13:859668. [60] DENG S, XIANG JJ, SHEN YY, et al. Effects of VEGF-Notch Signaling Pathway on Proliferation and Apoptosis of Bone Marrow MSC in Patients with Aplastic Anemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2019;27(6):1925-1932. [61] ZHANG X, LIU L, DOU C, et al. PPAR Gamma-Regulated MicroRNA 199a-5p Underlies Bone Marrow Adiposity in Aplastic Anemia. Mol Ther Nucleic Acids. 2019;17:678-687. [62] LI JP, WU KH, CHAO WR, et al. Alterations of mesenchymal stem cells on regulating Th17 and Treg differentiation in severe aplastic anemia. Aging (Albany NY). 2023;15(2):553-566. [63] LI H, WANG L, PANG Y, et al. In patients with chronic aplastic anemia, bone marrow-derived MSCs regulate the Treg/Th17 balance by influencing the Notch/RBP-J/FOXP3/RORγt pathway. Sci Rep. 2017; 7:42488. [64] LI J, YANG S, LU S, et al. Differential gene expression profile associated with the abnormality of bone marrow mesenchymal stem cells in aplastic anemia. PLoS One. 2012;7(11):e47764. [65] HUO J, ZHANG L, REN X, et al. Multifaceted characterization of the signatures and efficacy of mesenchymal stem/stromal cells in acquired aplastic anemia. Stem Cell Res Ther. 2020;11(1):59. [66] GONZAGA VF, WENCESLAU CV, VIEIRA DP, et al. Therapeutic Potential of Human Immature Dental Pulp Stem Cells Observed in Mouse Model for Acquired Aplastic Anemia. Cells. 2022;11(14):2252. [67] ZHANG J, ZHOU S, ZHOU Y, et al. Adipose-Derived Mesenchymal Stem Cells (ADSCs) With the Potential to Ameliorate Platelet Recovery, Enhance Megakaryopoiesis, and Inhibit Apoptosis of Bone Marrow Cells in a Mouse Model of Radiation-Induced Thrombocytopenia. Cell Transplant. 2016;25(2):261-273. [68] DIAZ MF, HORTON PD, DUMBALI SP, et al. Bone marrow stromal cell therapy improves survival after radiation injury but does not restore endogenous hematopoiesis. Sci Rep. 2020;10(1):22211. [69] FOUILLARD L, BENSIDHOUM M, BORIES D, et al. Engraftment of allogeneic mesenchymal stem cells in the bone marrow of a patient with severe idiopathic aplastic anemia improves stroma. Leukemia. 2003;17(2):474-476. [70] CLÉ DV, SANTANA-LEMOS B, TELLECHEA MF, et al. Intravenous infusion of allogeneic mesenchymal stromal cells in refractory or relapsed aplastic anemia. Cytotherapy. 2015;17(12):1696-1705. [71] PANG Y, XIAO HW, ZHANG H, et al. Allogeneic Bone Marrow-Derived Mesenchymal Stromal Cells Expanded In Vitro for Treatment of Aplastic Anemia: A Multicenter Phase II Trial. Stem Cells Transl Med. 2017;6(7):1569-1575. [72] LAN Y, LIU F, CHANG L, et al. Combination of umbilical cord mesenchymal stem cells and standard immunosuppressive regimen for pediatric patients with severe aplastic anemia. BMC Pediatr. 2021;21(1):102. [73] DING L, HAN DM, ZHENG XL, et al. A study of human leukocyte antigen-haploidentical hematopoietic stem cells transplantation combined with allogenic mesenchymal stem cell infusion for treatment of severe aplastic anemia in pediatric and adolescent patients. Stem Cells Transl Med. 2021;10(2):291-302. [74] LI T, LUO C, ZHANG J, et al. Efficacy and safety of mesenchymal stem cells co-infusion in allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis. Stem Cell Res Ther. 2021; 12(1):246. [75] YUAN F, LI M, WEI X, et al. Co-transplantation of umbilical cord mesenchymal stem cells and peripheral blood stem cells in children and adolescents with refractory or relapsed severe aplastic anemia. Pediatr Hematol Oncol. 2024;41(5):322-335. [76] 丁宇斌,唐玉凤,唐旭东.干细胞移植治疗重型再生障碍性贫血:研究应用与进展[J].中国组织工程研究,2020,24(19):3084-3092. [77] SHENG XF, LI H, HONG LL, et al. Combination of Haploidentical Hematopoietic Stem Cell Transplantation with Umbilical Cord-Derived Mesenchymal Stem Cells in Patients with Severe Aplastic Anemia: A Retrospective Controlled Study. Turk J Haematol. 2022;39(2):117-129. [78] 韩冬梅,丁丽,郑晓丽,等.单倍体造血干细胞移植联合间充质干细胞治疗重型再生障碍性贫血[J].中国实验血液学杂志,2022, 30(4):1230-1237. [79] AIZAWA K, PELTIER D, MATSUKI E, et al. How does transfusion-associated graft-versus-host disease compare to hematopoietic cell transplantation-associated graft-versus-host disease? Transfus Apher Sci. 2022;61(2):103405. [80] HILL GR, BETTS BC, TKACHEV V,et al. Current Concepts and Advances in Graft-Versus-Host Disease Immunology. Annu Rev Immunol. 2021; 39:19-49. [81] AKÇAY A, ATAY D, ERBEY F, et al. Safety and Efficacy of Co-transplantation of Hematopoietic Stem Cells Combined With Human Umbilical Cord-Derived Mesenchymal Stem Cells in Children With Severe Aplastic Anemia: A Single-Center Experience. Exp Clin Transplant. 2022;20(12):1114-1121. [82] DING L, HAN DM, ZHENG XL, et al. Infusion of haploidentical hematopoietic stem cells combined with mesenchymal stem cells for treatment of severe aplastic anemia in adult patients yields curative effects. Cytotherapy. 2022;24(2):205-212. [83] LI XH, GAO CJ, DA WM, et al. Reduced intensity conditioning, combined transplantation of haploidentical hematopoietic stem cells and mesenchymal stem cells in patients with severe aplastic anemia. PLoS One. 2014;9(3):e89666. [84] KEBRIAEI P, HAYES J, DALY A, et al. A Phase 3 Randomized Study of Remestemcel-L versus Placebo Added to Second-Line Therapy in Patients with Steroid-Refractory Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2020;26(5):835-844. [85] ZHAO L, CHEN S, YANG P, et al. The role of mesenchymal stem cells in hematopoietic stem cell transplantation: prevention and treatment of graft-versus-host disease. Stem Cell Res Ther. 2019;10(1):182. [86] 韩振霞,时庆,汪大琨,等.骨髓源与脐带源间充质干细胞的基本生物学特征比较[J].中国实验血液学杂志,2013,21(5):1248-1255. [87] STAB BR 2ND, MARTINEZ L, GRISMALDO A, et al. Mitochondrial Functional Changes Characterization in Young and Senescent Human Adipose Derived MSCs. Front Aging Neurosci. 2016;8:299. [88] SAREEN N, SEQUIERA GL, CHAUDHARY R, et al. Early passaging of mesenchymal stem cells does not instigate significant modifications in their immunological behavior. Stem Cell Res Ther. 2018;9(1):121. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Liu Lin, Liu Shixuan, Lu Xinyue, Wang Kan. Metabolomic analysis of urine in a rat model of chronic myofascial trigger points [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1585-1592. |
| [3] | Su Xiaoyang, Chen Wenting, Fu Yidan, Zhao Yan, Lan Danfeng, Yang Qiuping. Correlation between Mer receptor tyrosine kinase and diabetic peripheral neuropathy in Sprague-Dawley rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1593-1599. |
| [4] | Li Kaiying, Wei Xiaoge, Song Fei, Yang Nan, Zhao Zhenning, Wang Yan, Mu Jing, Ma Huisheng. Mechanism of Lijin manipulation regulating scar formation in skeletal muscle injury repair in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1600-1608. |
| [5] | Li Jun, Gong Jingjing, Sun Guobin, Guo Rui, Ding Yang, Qiang Lijuan, Zhang Xiaoli, Fang Zhanhai . miR-27a-3p promotes the proliferation of human hypertrophic scar fibroblasts by regulating mitogen-activated protein kinase signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1609-1617. |
| [6] | Li Huayuan, Li Chun, Liu Junwei, Wang Ting, Li Long, Wu Yongli. Effect of warm acupuncture on PINK1/Parkin pathway in the skeletal muscle of rats with chronic fatigue syndrome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1618-1625. |
| [7] | Jing Ruyi, Chen Yingxin, Cao Lei . Prognosis of deep lamellar keratoplasty versus penetrating keratoplasty in the treatment of stromal corneal dystrophy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1626-1633. |
| [8] | Wang Xuanqiang, Zhang Wenyang, Li Yang, Kong Weiqian, Li Wei, Wang Le, Li Zhongshan, Bai Shi. Effects of chronic exposure to low-frequency pulsed magnetic fields on contractility and morphology of the quadriceps muscle in healthy adults [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1634-1642. |
| [9] | Zhang Yuxin, Yu Cong, Zhang Cui, Ding Jianjun, Chen Yan. Differences in postural control ability between older adults with mild cognitive impairment and those with normal cognition under different single-task and dual-task conditions [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1643-1649. |
| [10] | Zhou Panpan, Cui Yinglin, Zhang Wentao, Wang Shurui, Chen Jiahui, Yang Tong . Role of cellular autophagy in cerebral ischemic injury and the regulatory mechanism of traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1650-1658. |
| [11] | Yu Jingbang, Wu Yayun. Regulatory effect of non-coding RNA in pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1659-1666. |
| [12] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [13] | Zhu Hanmin, Wang Song, Xiao Wenlin, Zhang Wenjing, Zhou Xi, He Ye, Li Wei, . Mitophagy regulates bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1676-1683. |
| [14] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [15] | Wang Yida, Liu Jun, Wang Xiaoling, Wang Liyan, Yang Chengru, Zhang Xuexiao. Effects of wearable electronic device-based interventions on physical activity and sedentary behavior in healthy adolescents: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1693-1704. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

