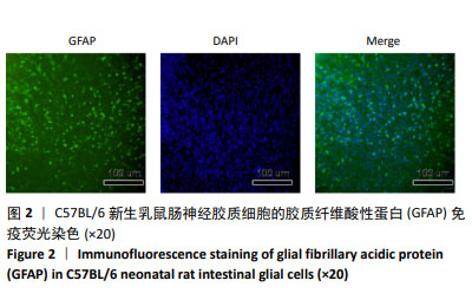
[1] VICENTINI FA, KEENAN CM, WALLACE LE, et al. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome. 2021 26;9(1):210.
[2] SINGH N, BERNSTEIN CN. Environmental risk factors for inflammatory bowel disease. United European Gastroenterol J. 2022;10(10): 1047-1053.
[3] KHOR B, GARDET A, XAVIER RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474(7351):307-317.
[4] LI H, FAN C, LU H, et al. Protective role of berberine on ulcerative colitis through modulating enteric glial cells-intestinal epithelial cells-immune cells interactions. Acta Pharm Sin B. 2020;10(3):447-461.
[5] SCHNEIDER KM, BLANK N, ALVAREZ Y, et al. The enteric nervous system relays psychological stress to intestinal inflammation. Cell. 2023; 186(13):2823-2838.e20.
[6] SUMAN S. Enteric Nervous System Alterations in Inflammatory Bowel Disease: Perspectives and Implications. Gastrointest Disord (Basel). 2024;6(2):368-379.
[7] LE BERRE C, NAVEILHAN P, ROLLI-DERKINDEREN M. Enteric glia at center stage of inflammatory bowel disease. Neurosci Lett. 2023;809:137315.
[8] THOMASI B, GULBRANSEN B. Mini-review: Intercellular communication between enteric glia and neurons. Neurosci Lett. 2023;806:137263.
[9] SHARKEY KA, MAWE GM. The enteric nervous system. Physiol Rev. 2023;103(2):1487-1564.
[10] SCHNEIDER KM, KIM J, BAHNSEN K, et al. Environmental perception and control of gastrointestinal immunity by the enteric nervous system. Trends Mol Med. 2022;28(11):989-1005.
[11] AHMADZAI MM, SEGUELLA L, GULBRANSEN BD. Circuit-specific enteric glia regulate intestinal motor neurocircuits. Proc Natl Acad Sci U S A. 2021;118(40):e2025938118.
[12] LE BERRE C, NAVEILHAN P, ROLLI-DERKINDEREN M. Enteric glia at center stage of inflammatory bowel disease. Neurosci Lett. 2023;809:137315.
[13] RAO M, GULBRANSEN BD. Enteric Glia. Cold Spring Harb Perspect Biol. 2024:a041368.
[14] THOMASI B, VALDETARO L, RICCIARDI MC, et al. Enteric glia as a player of gut-brain interactions during Parkinson’s disease. Front Neurosci. 2023;17:1281710.
[15] THOMASI B, VALDETARO L, GULBRANSEN B, et al. Neuroimmune Connectomes in the Gut and Their Implications in Parkinson’s Disease. Mol Neurobiol. 2024;61(4):2081-2098.
[16] SUNARDI M, CIRILLO C. Mini-review: “Enteric glia functions in nervous tissue repair: Therapeutic target or tool?”. Neurosci Lett. 2023;812: 137360.
[17] ZIEGLER AL, CALDWELL ML, CRAIG SE, et al. Enteric glial cell network function is required for epithelial barrier restitution following intestinal ischemic injury in the early postnatal period. Am J Physiol Gastrointest Liver Physiol. 2024;326(3):G228-G246.
[18] WALLRAPP A, YANG D, CHIU IM. Enteric glial cells mediate gut immunity and repair. Trends Neurosci. 2022;45(4):251-253.
[19] ZHANG X, QI F, YANG J, et al. Distribution and ultrastructural characteristics of enteric glial cell in the chicken cecum. Poult Sci. 2024;103(10):104070.
[20] SCHONKEREN SL, KÜTHE TT, IDRIS M, et al. The gut brain in a dish: Murine primary enteric nervous system cell cultures. Neurogastroenterol Motil. 2022;34(2):e14215.
[21] TERAMOTO H, HIRASHIMA N, TANAKA M. A Simple Method for Purified Primary Culture of Enteric Glial Cells from Mouse Small Intestine. Biol Pharm Bull. 2022;45(4):547-551.
[22] ZANOLETTI L, VALDATA A, NEHLSEN K, et al. Cytological, molecular, cytogenetic, and physiological characterization of a novel immortalized human enteric glial cell line. Front Cell Neurosci. 2023;17:1170309.
[23] SMITH TH, NGWAINMBI J, GRIDER JR, et al. An in-vitro preparation of isolated enteric neurons and glia from the myenteric plexus of the adult mouse. J Vis Exp. 2013;(78):50688.
[24] WANG Z, OCADIZ-RUIZ R, SUNDARESAN S, et al. Isolation of Enteric Glial Cells from the Submucosa and Lamina Propria of the Adult Mouse. J Vis Exp. 2018;(138):57629.
[25] WINDSTER JD, SACCHETTI A, SCHAAF GJ, et al. A combinatorial panel for flow cytometry-based isolation of enteric nervous system cells from human intestine. EMBO Rep. 2023;24(4):e55789.
[26] 李娜,高慧,张煜鑫,等.胚胎小鼠肠神经胶质细胞的分离与鉴定[J].神经解剖学杂志,2019,35(6):636-640.
[27] SCAVUZZO MA, LETAI KC, MAENO-HIKICHI Y, et al. Enteric glial hub cells coordinate intestinal motility. bioRxiv [Preprint]. 2023:2023. 06.07.544052.
[28] SANTHOSH S, ZANOLETTI L, STAMP LA, et al. From diversity to disease: unravelling the role of enteric glial cells. Front Immunol. 2024;15: 1408744.
[29] WANG F, LI N, WEI X, et al. MRI-Guided Focused Ultrasound-Induced Blood Brain Barrier Disruption to Deliver Glial Cell Line Derived Neurotropic Factor Proteins into Brain to Treat Rat Depression. J Biomed Nanotechnol. 2020;16(5):626-639.
[30] CERANTOLA S, CAPUTI V, MARSILIO I, et al. Involvement of Enteric Glia in Small Intestine Neuromuscular Dysfunction of Toll-Like Receptor 4-Deficient Mice. Cells. 2020;9(4):838.
[31] HE Y, HARA H, NÚÑEZ G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci. 2016;41(12):1012-1021.
[32] NEUNLIST M, ROLLI-DERKINDEREN M, LATORRE R, et al. Enteric glial cells: recent developments and future directions. Gastroenterology. 2014;147(6):1230-1237.
[33] SHARKEY KA. Emerging roles for enteric glia in gastrointestinal disorders. J Clin Invest. 2015;125(3):918-925.
[34] REALE O, BODI D, HUGUET A, et al. Role of enteric glial cells in the toxicity of phycotoxins: Investigation with a tri-culture intestinal cell model. Toxicol Lett. 2021;351:89-98.
[35] WANG X, WU J, LIU X, et al. Engineered liposomes targeting the gut-CNS Axis for comprehensive therapy of spinal cord injury. J Control Release. 2021;331:390-403.
[36] SCHULTE S, DECKER D, NOWDURI B, et al. Improving morphological and functional properties of enteric neuronal networks in vitro using a novel upside-down culture approach. Am J Physiol Gastrointest Liver Physiol. 2024;326(5):G567-G582.
[37] LEFÈVRE MA, SORET R, PILON N. Harnessing the Power of Enteric Glial Cells’ Plasticity and Multipotency for Advancing Regenerative Medicine. Int J Mol Sci. 2023;24(15):12475.
[38] BAGHDADI MB, KIM TH. The multiple roles of enteric glial cells in intestinal homeostasis and regeneration. Semin Cell Dev Biol. 2023; 150-151:43-49.
[39] DRUMM BT, COBINE CA, BAKER SA. Insights on gastrointestinal motility through the use of optogenetic sensors and actuators. J Physiol. 2022; 600(13):3031-3052.
[40] DURAND T, PAUL-GILLOTEAUX P, GORA M, et al. Visualizing enteric nervous system activity through dye-free dynamic full-field optical coherence tomography. Commun Biol. 2023;6(1):236. |