Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (22): 4653-4662.doi: 10.12307/2025.443
Previous Articles Next Articles
Chondrocyte proliferation and tissue formation enhanced by stromal cell derived factor-1 modified poly-L-lactic acid porous microspheres
Ma Yue, Tan Shiyu, Chu Feiyang, Chen Zhuoqi, Liu Siyu, Liu Wenshuai, Liu Xia
- Research Center of Plastic Surgery Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100144, China
-
Received:2024-03-07Accepted:2024-05-17Online:2025-08-08Published:2024-12-05 -
Contact:Liu Wenshuai, Assistant researcher, Research Center of Plastic Surgery Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100144, China Liu Xia, Researcher, Research Center of Plastic Surgery Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100144, China -
About author:Ma Yue, Master, Research Center of Plastic Surgery Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100144, China -
Supported by:National Natural Science Foundation of China (General Program), No. 81871575 (to LX); Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, No. 2021-I2M-1-052 (to LX, LWS)
CLC Number:
Cite this article
Ma Yue, Tan Shiyu, Chu Feiyang, Chen Zhuoqi, Liu Siyu, Liu Wenshuai, Liu Xia. Chondrocyte proliferation and tissue formation enhanced by stromal cell derived factor-1 modified poly-L-lactic acid porous microspheres[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(22): 4653-4662.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
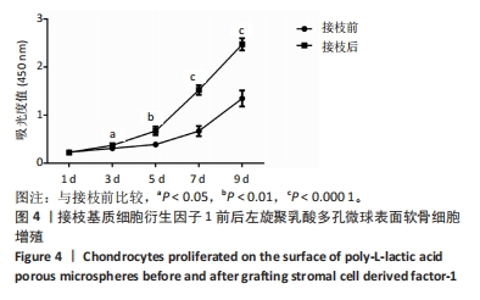
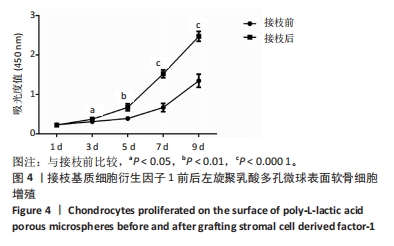
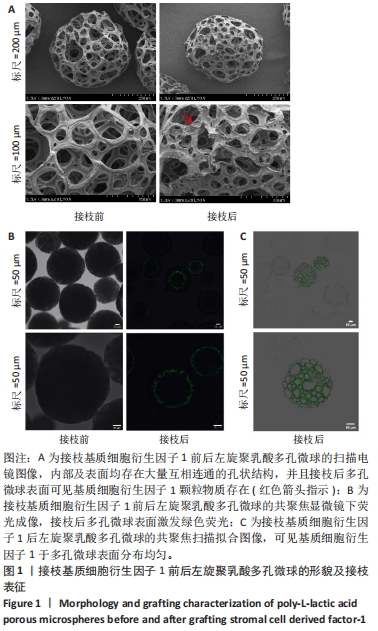
2.1 左旋聚乳酸多孔微球的形貌 扫描电镜下可见接枝与未接枝基质细胞衍生因子1左旋聚乳酸多孔微球内部及表面均存在大量互相连通的孔状结构,并且接枝基质细胞衍生因子1左旋聚乳酸表面有颗粒样物质存在(箭头所示),见图1A。未接枝基质细胞衍生因子1左旋聚乳酸多孔微球的孔隙率为90.63%,接枝基质细胞衍生因子1左旋聚乳酸多孔微球的孔隙率为89.83%。 2.2 基质细胞衍生因子1接枝表征 接枝基质细胞衍生因子1左旋聚乳酸多孔微球在不同层面扫描时均激发绿色荧光,见图1B。最终将荧光图像层层扫描后拟合输出,可见基质细胞衍生因子1均匀分布于微球,几乎遍布多孔微球表面,见图1C。利用基质细胞衍生因子1 ELISA试剂盒测量接枝完成后上清液中未成功接枝的因子量,通过间接法得到基质细胞衍生因子1 接枝率约为93.75%,与图1C中绿色荧光拟合图像相符,均验证了良好的接枝效果。"
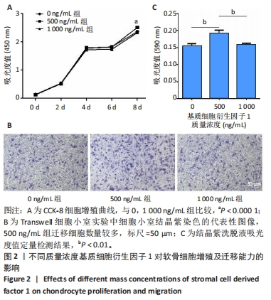
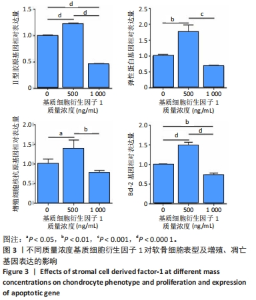
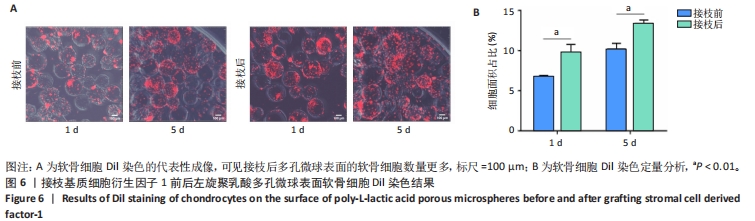
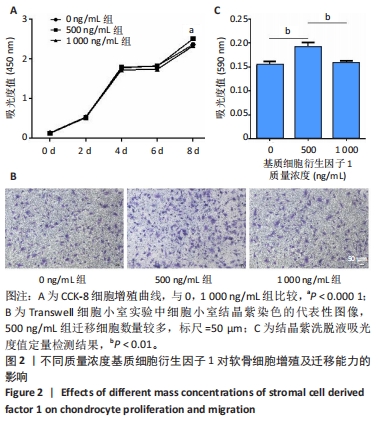
2.3 基质细胞衍生因子1对软骨细胞增殖、迁移、软骨表型维持的影响 CCK-8检测结果显示,随着培养时间的延长,各组细胞数量逐渐增加,培养第8天,500 ng/mL 基质细胞衍生因子1组细胞增殖吸光度值高于0,1 000 ng/mL 基质细胞衍生因子1组(P < 0.000 1),见图2A,说明添加500 ng/mL基质细胞衍生因子1对软骨细胞增殖具有促进作用。 Transwell细胞小室实验结果显示,500 ng/mL 基质细胞衍生因子1组细胞迁移能力强于0,1 000 ng/mL 基质细胞衍生因子1组(P < 0.01),见图2B,C,说明500 ng/mL基质细胞衍生因子1可促进软骨细胞的迁移。"
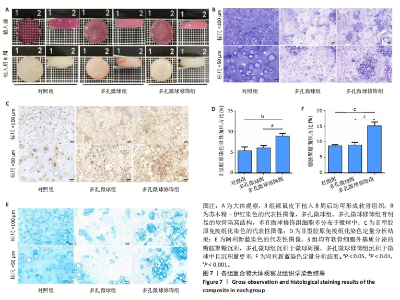
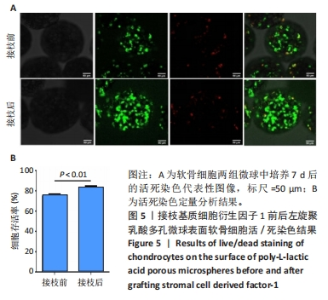
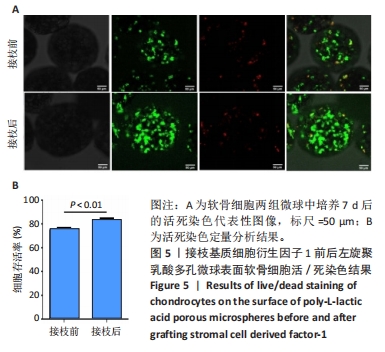
2.5 左旋聚乳酸多孔微球植入裸鼠皮下后体内成软骨检测 2.5.1 实验动物数量分析 12只裸鼠全部进入结果分析。 2.5.2 各组复合物大体观察 对照组、多孔微球组、多孔微球修饰组的组织块大小及厚度无明显差别,形态维持良好,3组组织块硬度手感无明显差异,均可生成乳白色软骨样组织,见图7A。 2.5.3 各组复合物组织学染色结果 苏木精-伊红染色结果显示,对照组软骨细胞散在分布于甲基丙烯酰胺基明胶中,相比之下,多孔微球组及多孔微球修饰组软骨细胞更倾向于聚集分布,多孔微球修饰组中有更多的细胞黏附、生长于多孔微球中,见图7B。Ⅱ型胶原免疫组化染色结果显示,多孔微球修饰组软骨细胞Ⅱ型胶原分泌多于对照组(P < 0.01)、多孔微球组(P < 0.05),见图7C,D。阿利新蓝染色结果显示,多孔微球修饰组软骨细胞的细胞外基质糖胺聚糖沉积多于对照组、多孔微球组(P均< 0.001),见图7E,F。"

| [1] STAMPOULTZIS T, KARAMI P, PIOLETTI DP. Thoughts on cartilage tissue engineering: A 21st century perspective. Curr Res Transl Med. 2021;69(3):103299. [2] BALUCH N, NAGATA S, PARK C, et al. Auricular reconstruction for microtia: A review of available methods. Plast Surg (Oakv). 2014; 22(1):39-43. [3] CHEN FM, LIU X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci. 2016;53:86-168. [4] RASTOGI P, KANDASUBRAMANIAN B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication. 2019;11(4):42001. [5] SALEHI S, KOECK K, SCHEIBEL T. Spider Silk for Tissue Engineering Applications. Molecules. 2020;25(3):737. [6] CATOIRA MC, FUSARO L, DI FRANCESCO D, et al. Overview of natural hydrogels for regenerative medicine applications. J Mater Sci Mater Med. 2019;30(10):115. [7] WU DT, MUNGUIA-LOPEZ JG, CHO YW, et al. Polymeric Scaffolds for Dental, Oral, and Craniofacial Regenerative Medicine. Molecules. 2021;26(22):7043. [8] LEE HI, HEO Y, BAEK SW, et al. Multifunctional Biodegradable Vascular PLLA Scaffold with Improved X-ray Opacity, Anti-Inflammation, and Re-Endothelization. Polymers (Basel). 2021;13(12):1979. [9] REDDY M, PONNAMMA D, CHOUDHARY R, et al. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers (Basel). 2021;13(7):1105. [10] XIAO G, YIN H, XU W, et al. Modification and cytocompatibility of biocomposited porous PLLA/HA-microspheres scaffolds. J Biomater Sci Polym Ed. 2016;27(14):1462-1475. [11] RAVI M, PARAMESH V, KAVIYA SR, et al. 3D cell culture systems: advantages and applications. J Cell Physiol. 2015;230(1):16-26. [12] KANG SW, SEO SW, CHOI CY, et al. Porous poly(lactic-co-glycolic acid) microsphere as cell culture substrate and cell transplantation vehicle for adipose tissue engineering. Tissue Eng Part C Methods. 2008;14(1):25-34. [13] LIN A, LIU S, XIAO L, et al. Controllable preparation of bioactive open porous microspheres for tissue engineering. J Mater Chem B. 2022;10(34):6464-6471. [14] ZENG Y, LI X, LIU X, et al. PLLA Porous Microsphere-Reinforced Silk-Based Scaffolds for Auricular Cartilage Regeneration. ACS Omega. 2021;6(4):3372-3383. [15] LIU S, WANG YN, MA B, et al. Gingipain-Responsive Thermosensitive Hydrogel Loaded with SDF-1 Facilitates In Situ Periodontal Tissue Regeneration. ACS Appl Mater Interfaces. 2021;13(31):36880-36893. [16] WANG Y, SUN X, LV J, et al. Stromal Cell-Derived Factor-1 Accelerates Cartilage Defect Repairing by Recruiting Bone Marrow Mesenchymal Stem Cells and Promoting Chondrogenic Differentiation<sup/>. Tissue Eng Part A. 2017;23(19-20):1160-1168. [17] LI J, CHEN H, ZHANG D, et al. The role of stromal cell-derived factor 1 on cartilage development and disease. Osteoarthritis Cartilage. 2021;29(3):313-322. [18] YUE K, TRUJILLO-DE SG, ALVAREZ MM, et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254-271. [19] LU W, ZENG M, LIU W, et al. Human urine-derived stem cell exosomes delivered via injectable GelMA templated hydrogel accelerate bone regeneration. Mater Today Bio. 2023;19:100569. [20] JIN C, XU G. Study on the Promotion of hADSCs Migration and Chemotaxis by SDF-1. Asia Pac J Ophthalmol (Phila). 2023;12(3):303-309. [21] CHIESA-ESTOMBA CM, HERNAEZ-MOYA R, RODINO C, et al. Ex Vivo Maturation of 3D-Printed, Chondrocyte-Laden, Polycaprolactone-Based Scaffolds Prior to Transplantation Improves Engineered Cartilage Substitute Properties and Integration. Cartilage. 2022;13(4):105-118. [22] LI X, LI X, YANG J, et al. Living and Injectable Porous Hydrogel Microsphere with Paracrine Activity for Cartilage Regeneration. Small. 2023;19(17):e2207211. [23] TAN YJ, TAN X, YEONG WY, et al. Hybrid microscaffold-based 3D bioprinting of multi-cellular constructs with high compressive strength: A new biofabrication strategy. Sci Rep. 2016;6:39140. [24] BIJU TS, PRIYA VV, FRANCIS AP. Role of three-dimensional cell culture in therapeutics and diagnostics: an updated review. Drug Deliv Transl Res. 2023;13(9):2239-2253. [25] JIN GZ, KIM HW. Porous microcarrier-enabled three-dimensional culture of chondrocytes for cartilage engineering: A feasibility study. Tissue Eng Regen Med. 2016;13(3):235-241. [26] GUPTA V, KHAN Y, BERKLAND CJ, et al. Microsphere-Based Scaffolds in Regenerative Engineering. Annu Rev Biomed En. 2017;19:135-161. [27] YUAN X, YANG W, FU Y, et al. Four-Arm Polymer-Guided Formation of Curcumin-Loaded Flower-Like Porous Microspheres as Injectable Cell Carriers for Diabetic Wound Healing. Adv Healthc Mater. 2023; 12(30):e2301486. [28] QIAO Z, ZHANG W, JIANG H, et al. 3D-printed composite scaffold with anti-infection and osteogenesis potential against infected bone defects. RSC Adv. 2022;12(18):11008-11020. [29] PAN Q, SU W, YAO Y. Progress in microsphere-based scaffolds in bone/cartilage tissue engineering. Biomed Mater. 2023;18(6). doi: 10.1088/1748-605X/acfd78. [30] FINKLEA FB, TIAN Y, KERSCHER P, et al. Engineered cardiac tissue microsphere production through direct differentiation of hydrogel-encapsulated human pluripotent stem cells. Biomaterials. 2021;274: 120818. [31] DENG S, ZHAO X, ZHU Y, et al. Efficient hepatic differentiation of hydrogel microsphere-encapsulated human pluripotent stem cells for engineering prevascularized liver tissue. Biofabrication. 2022;15(1). doi: 10.1088/1758-5090/aca79b. [32] GHUMAN H, MATTA R, TOMPKINS A, et al. ECM hydrogel improves the delivery of PEG microsphere-encapsulated neural stem cells and endothelial cells into tissue cavities caused by stroke. Brain Res Bull. 2021;168:120-137. [33] YANG Y, YAO X, LI X, et al. Non-mulberry silk fiber-based scaffolds reinforced by PLLA porous microspheres for auricular cartilage: An in vitro study. Int J Biol Macromol. 2021;182:1704-1712. [34] HUANG L, XIAO L, JUNG PA, et al. Porous chitosan microspheres as microcarriers for 3D cell culture. Carbohydr Polym. 2018;202:611-620. [35] WANG SQ, XIA J, CHEN J, et al. Influence of biological scaffold regulation on the proliferation of chondrocytes and the repair of articular cartilage. Am J Transl Res. 2016;8(11):4564-4573. [36] ZHANG Y, CHOI SW, XIA Y. Modifying the pores of an inverse opal scaffold with chitosan microstructures for truly three-dimensional cell culture. Macromol Rapid Commun. 2012;33(4):296-301. [37] LI Y, XIAO Y, LIU C. The Horizon of Materiobiology: A Perspective on Material-Guided Cell Behaviors and Tissue Engineering. Chem Rev. 2017;117(5):4376-4421. [38] LUO Y, XIAO M, ALMAQRAMI BS, et al. Regenerated silk fibroin based on small aperture scaffolds and marginal sealing hydrogel for osteochondral defect repair. Biomater Res. 2023;27(1):50. [39] 黄元亮,穆琳,林燕娴,等.丝素蛋白-左旋聚乳酸微载体扩增脂肪来源干细胞的实验研究[J].中国修复重建外科杂志,2021,35(5):611-619. [40] LI Q, CHANG B, DONG H, et al. Functional microspheres for tissue regeneration. Bioact Mater. 2023;25:485-499. [41] BAI L, HAN Q, HAN Z, et al. Stem Cells Expansion Vector via Bioadhesive Porous Microspheres for Accelerating Articular Cartilage Regeneration. Adv Healthc Mater. 2024;13(3):e2302327. [42] THAKUR G, RODRIGUES FC, SINGH K. Crosslinking Biopolymers for Advanced Drug Delivery and Tissue Engineering Applications. Adv Exp Med Biol. 2018;1078:213-231. [43] NAIR M, JOHAL RK, HAMAIA SW, et al. Tunable bioactivity and mechanics of collagen-based tissue engineering constructs: A comparison of EDC-NHS, genipin and TG2 crosslinkers. Biomaterials. 2020;254:120109. [44] GRABSKA-ZIELINSKA S, SIONKOWSKA A, CARVALHO A, et al. Biomaterials with Potential Use in Bone Tissue Regeneration-Collagen/Chitosan/Silk Fibroin Scaffolds Cross-Linked by EDC/NHS. Materials (Basel). 2021;14(5):1105. [45] LI L X, ZHANG XF, BAI X, et al. SDF-1 promotes ox-LDL induced vascular smooth muscle cell proliferation. Cell Biol Int. 2013;37(9):988-994. [46] SADRI F, REZAEI Z, FEREIDOUNI M. The significance of the SDF-1/CXCR4 signaling pathway in the normal development. Mol Biol Rep. 2022;49(4):3307-3320. |
| [1] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [2] | Yin Lu, Jiang Chuanfeng, Chen Junjie, Yi Ming, Wang Zihe, Shi Houyin, Wang Guoyou, Shen Huarui. Effect of Complanatoside A on the apoptosis of articular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1541-1547. |
| [3] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [4] | Yang Zhihang, Sun Zuyan, Huang Wenliang, Wan Yu, Chen Shida, Deng Jiang. Nerve growth factor promotes chondrogenic differentiation and inhibits hypertrophic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1336-1342. |
| [5] | Xiang Pan, Che Yanjun, Luo Zongping. Compressive stress induces degeneration of cartilaginous endplate cells through the SOST/Wnt/beta-catenin pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 951-957. |
| [6] | Yu Shuangqi, Ding Fan, Wan Song, Chen Wei, Zhang Xuejun, Chen Dong, Li Qiang, Lin Zuoli. Effects of polylactic acid-glycolic acid copolymer/lysine-grafted graphene oxide nanoparticle composite scaffolds on osteogenic differentiation of MC3T3 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 707-712. |
| [7] | Ma Weibang, Xu Zhe, Yu Qiao, Ouyang Dong, Zhang Ruguo, Luo Wei, Xie Yangjiang, Liu Chen. Screening and cytological validation of cartilage degeneration-related genes in exosomes from osteoarthritis synovial fluid [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7783-7789. |
| [8] | Sima Xinli, Liu Danping, Qi Hui. Effect and mechanism of metformin-modified bone marrow mesenchymal stem cell exosomes on regulating chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7728-7734. |
| [9] | Yin Hang, Song Kui. Effect of crocin hydrogel on chondrocytes and MC3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7293-7300. |
| [10] | Yu Qinghe, Cai Ziming, Wu Jintao, Ma Pengfei, Zhang Xin, Zhou Longqian, Wang Yakun, Lin Xiaoqin, Lin Wenping. Vanillic acid inhibits inflammatory response and extracellular matrix degradation of endplate chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6391-9397. |
| [11] | An Xingqi, Li Wenjin. Mechanism of Notch signaling pathway regulating condylar cartilage development and temporomandibular joint inflammation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5673-5679. |
| [12] | Liang Zhifeng, Yang Yingcai, Cheng Qiangang, Jia Yongxing, Wang Bo . Effect of stromal cell-derived factor-1 in cartilage and subchondral bone homeostasis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5422-5433. |
| [13] | Zheng Li, Ding Yiheng, Li Xinhao, Wen Zekai, Jiang Bingzheng, Lin Xuexia. Role of neuropilin 1 in promoting angiogenesis-osteogenesis coupling during fracture healing [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(21): 4576-4583. |
| [14] | Yang Dujuan, Cheng Mengke, Liu Jia. Osteogenic/odontogenic differentiation ability of human dental pulp stem cells under photocrosslinked composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 4022-4028. |
| [15] | Wang Zhe, Qi Yansong, Xu Yongsheng. Diagnosis and treatment of osteoarthritis with exosomes derived from different stem cells and carrying non-coding RNA [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 4122-4131. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||