Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (34): 7423-7430.doi: 10.12307/2025.500
Previous Articles Next Articles
Application of biodegradable magnesium and magnesium-based materials in oral diseases
Zhao Cong1, Fu Huiyuan1, Su Nite1, Ji Jie1, Zhang Lei1, Wang Yaxian2
- 1Hohhot Stomatological Hospital (Inner Mongolia Autonomous Region Stomatological Hospital), Hohhot 010000, Inner Mongolia Autonomous Region, China; 2Ningbo Branch of Chinese Academy of Weapons Science, Ningbo 315000, Zhejiang Province, China
-
Received:2024-07-18Accepted:2024-09-14Online:2025-12-08Published:2025-01-17 -
Contact:Wang Yaxian, MS, Assistant researcher, Ningbo Branch of Chinese Academy of Weapons Science, Ningbo 315000, Zhejiang Province, China -
About author:Zhao Cong, MS, Physician, Hohhot Stomatological Hospital (Inner Mongolia Autonomous Region Stomatological Hospital), Hohhot 010000, Inner Mongolia Autonomous Region, China -
Supported by:Northern Institute of Materials Science and Engineering Science and Technology Innovation Fund Project, No. NBFJ2019-cly2 (to WYX)
CLC Number:
Cite this article
Zhao Cong, Fu Huiyuan, Su Nite, Ji Jie, Zhang Lei, Wang Yaxian. Application of biodegradable magnesium and magnesium-based materials in oral diseases[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7423-7430.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
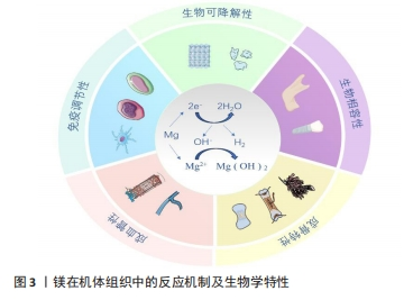
2.2.2 生物可降解性 镁及镁基材料植入后会在人体体液环境中发生降解,产生氢氧化镁、氢气和其他降解产物(图3),其中氢氧化物会作为保护涂层附着于植入物表面,以防植入物进一步腐蚀降解[17]。人体内的巨噬细胞会吞噬部分降解产物,其余降解产物存留于骨基质中,储存在骨基质中的镁离子进一步与羟基磷灰石晶体结合,提高了羟基磷灰石晶体的溶解度,进而增加了骨强度[18]。在镁合金缓慢降解后,暂时积聚的镁离子可通过局部骨再生及循环系统释放,而不会引起血清镁离子水平的波动。正是由于这种生物可降解特性,当镁作为骨折固定物时避免了二次手术取出的风险。虽然纯镁在体内的降解产物无毒,降解产物镁离子等也能促进骨再生,但镁基植入物在体内降解过快容易导致骨折治疗失败,因此,提高镁合金植入物的耐腐蚀性是延长镁合金植入物体内固定时间的关键[19]。有研究表明,镁的降解伴随着镁离子和氢气的持续释放,增加材料表面微环境中pH值和渗透压,这些近表面效应也可能会影响残留的肿瘤细胞,具有潜在的抗肿瘤活性,如骨肉瘤的治疗[20]。"

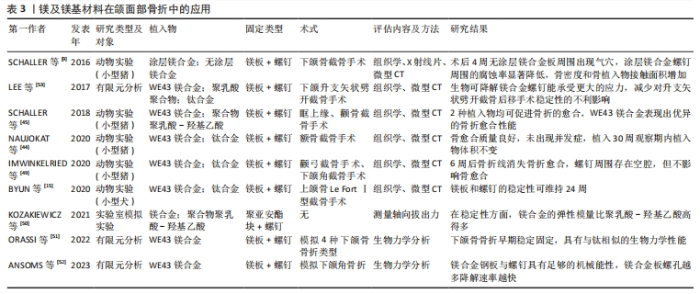
2.2.3 生物相容性 镁具有良好的生物相容性,植入后能与周围组织紧密接触且无不良反应,金属镁的密度为1.74-1.84 g/cm3,弹性模量为41-45 GPa,接近人体骨组织的密度和弹性模量(弹性模量为15-25 GPa)[21],这些性能为镁及镁合金作为骨植入材料提供了良好的应用基础。另外,镁及镁合金降解产生的镁离子也不会对机体造成负担,因为细胞可以耐受比生理浓度大16倍的镁离子浓度。动物实验结果表明,经等离子体电解氧化锂的镁锌钙合金植入兔颅骨缺损部位后以2.32 mm/年的速率降解,没有引起兔的心、肝、肾、脾功能障碍[22]。在植入WE43镁螺钉植入治疗骨折的临床试验也未发现高镁血症的迹象,并且血中镁浓度水平正常[23]。在正颌手术患者中应用WE43镁压缩螺钉后未观察到过敏反应、肝肾功能障碍或镁血清水平升高等并发症[24]。 2.2.4 成骨特性 与传统骨内植入物(如钛、钛合金、钴铬合金)相比,镁是软组织和骨组织中的重要元素,参与许多生化反应。有研究建议健康成人每日摄入310-420 mg镁离子以维持正常的生命活动,储存24-30 g镁离子以维持基础代谢[25]。人体内的大部分镁离子储存在骨组织中,如果骨组织中的镁离子含量降低,骨组织的力学性能就会减弱,导致骨小梁断裂,增加骨折风险。即使过量的镁离子被摄入体内也可以迅速地通过循环和泌尿系统排出体外,不会造成任何损害[26]。DíAZ-TOCADOS等[27]研究证明,增加镁离子浓度可增强体外间充质干细胞的成骨作用,镁离子还通过激活Notch信号通路增强成碱性磷酸酶、Runx2、骨钙素mRNA的表达。HUNG等[28]研究发现,含10 mmol/L镁离子的细胞培养基可有效促进骨髓间充质干细胞的成骨分化,通过激活Wnt信号通路增加细胞外基质中的矿化和碱性磷酸酶表达。WANG等[29]研究证明,镁离子通过有丝分裂原活化蛋白激酶信号通路促进骨髓间充质干细胞中成骨分化标志物的表达,如骨形态发生蛋白2、Ⅰ型胶原蛋白、Runx2。有研究发现,镁提取物促进了小鼠胚胎成骨细胞前体细胞MC3T3-E1的增殖和血小板衍生生长因子分泌,从而促进了成骨细胞发育和骨形成[30]。此外,镁还促进脂肪源性基质细胞骨桥蛋白基因的表达,骨桥蛋白是早期生物材料细胞成骨相互作用的必需基因。镁离子能促进各种成骨细胞的成骨分化,这是镁应用于骨内金属植入物的基本理论依据。破骨细胞生成和成骨之间的双向平衡在骨再生中起着核心作用[31]。除了调节成骨细胞促进骨再生外,镁还可以抑制破骨细胞的生成和骨吸收[32]。 2.2.5 成血管性 对于颌面部组织重建而言,构建功能化的血管网络极其重要[33]。血管网络参与骨再生一般有膜内和软骨内骨化2种方式。在膜内骨化过程中,血管网络延伸到骨膜的间充质区域诱导分化出成骨细胞[34];在软骨内骨化过程中,软骨模板中的非增殖软骨细胞分泌促血管生成因子以刺激血管生长,随着血管系统的扩张,软骨组织逐渐被骨组织取代,导致新骨的形成[35]。因此,促进体内血管化对骨再生至关重要。镁可调节多种内皮细胞的生物学功能,有研究表明,人脐静脉内皮细胞在一定量的镁离子中表现出高增殖率、良好的细胞形态和活力[36]。此外,一定浓度的镁离子也可以增加血管生成标志物的表达水平。YU等[10]采用等离子体浸没离子注入技术制备了一种潜在的牙种植体材料——双锌/镁离子共植入钛,以改善种植体的骨结合和长期生存,体外结果表明该系统中的镁离子通过上调人脐静脉内皮细胞中人镁离子转运蛋白的表达增加镁离子内流,通过激活缺氧诱导因子1α刺激血管内皮生长因子和血管内皮生长因子受体2的转录,从而诱导血管生成[37]。 2.2.6 免疫调节性 在骨组织工程中生物材料的骨免疫调节特性尤其受到重视,它影响着骨再生结果。免疫系统和骨骼系统共享着各种细胞因子受体和转录因子。巨噬细胞作为免疫系统的重要组成部分,包括M1型和M2型,M1型参与急性炎症,与组织修复的早期阶段密切相关;M2型在骨愈合后期发挥作用,导致主动修复或纤维组织的形成[38]。M1型和M2型巨噬细胞可以响应于各种生物材料而相互转换。巨噬细胞亚型转换过程可释放出各种成骨相关分子参与新骨形成。镁及镁基材料作为骨内金属植入物对成骨和破骨细胞有很大影响。当镁和镁合金植入体内后植入物周围的免疫反应会被触发,来自植入物的镁离子通过增强巨噬细胞向M2型极化来调节骨免疫微环境,M2型巨噬细胞分泌白细胞介素10以启动骨修复过程。此外,镁离子还可抑制M1巨噬细胞,降低促炎因子(肿瘤坏死因子α、白细胞介素6)的表达,从而起到抗炎作用。研究表明,镁合金植入后5 d和10 d显著刺激种植体-骨界面的巨噬细胞出现极化[39]。 综上所述,可降解镁基材料具有广泛的生物学活性,为其在口腔医学领域的临床应用奠定了基础。然而在免疫调节方面,目前研究仅集中于巨噬细胞所参与的组织反应,而对单核细胞、肥大细胞、T细胞和B细胞等的研究相对缺乏,以期日后进一步深入研究,完善相关免疫机制。同时,有部分学者提到的镁基材料抗肿瘤机制亦不清晰,相关详尽研究均待日后深入挖掘开展。 2.3 镁及镁基材料在口腔医学中的应用 2.3.1 镁及镁基材料在颌面部骨折中的应用 颌面部骨折大多由交通事故和暴力因素导致,对患者的外观容貌、言语和咀嚼功能都有显著影响。目前切开复位内固定是治疗颌面部骨折最常用的方法,使用的骨内固定材料多为不可降解金属,如广泛应用于临床的钛合金,尽管它具有良好的生物相容性、高耐磨性和高机械强度,但仍有一定的局限性,如可能发生暴露、感染、植入物松动及异物感等并发症,发生概率高达3%-25%[40],并且通常还需要二次手术取出,额外的手术也无疑增加了患者的痛苦和经济负担。之后有学者发现可降解合成聚合物如聚乳酸和聚乙醇酸可作为骨内固定物使用,并解决上述问题。然而多项研究证实,合成聚合物降解可产生有毒的残留单体、磨损碎片和积累酸性中间产物,这些产物可能导致病理性骨吸收,并且这类合成聚合物的强度比钛合金弱得多,因此临床使用受到限制。纯镁在人体组织内可发生快速降解并产生氢气,不仅无法有效固定骨折断端,还可能引起周围组织气肿,但通过加入其他金属元素及表面改性技术可提高镁基合金的耐腐蚀性和生物相容性,有效解决这一问题[41-43]。 NAUJOKAT等[44]在小型猪实验中发现,WE43涂层镁合金固定面中部骨折取得了可喜效果,30周内未发生明显的降解。SCHALLER等[45]在猪眶上缘和颧骨面中截骨手术中使用WE43镁合金或聚乳酸-羟基乙酸植入物固定后,与合成聚合物聚乳酸-羟基乙酸植入物相比,镁合金具有更小的植入物尺寸和更高的机械性能,可在负载环境中支持骨折愈合。LEONHARDT等[46]与KOZAKIEWICZ等[47]对可降解镁基加压螺钉植入治疗下颌髁突骨折的临床随访观察显示,镁合金植入物固定1年后的下颌骨髁突骨质愈合良好,颞下颌关节活动度好,张口受限明显改善,虽螺钉周围显示有气体产生但无需取出。由于固定颌面部骨折时大多采用口内入路,这就要求用于颌面区域的可生物降解材料必须克服该区域特有的因素,包括附着于颌面部不同骨骼的咀嚼肌力量、唾液的特有生理环境和口腔内病原微生物的存在。镁基材料具有良好的机械强度和生物相容性,临床应用已得到证实。然而,镁基合金的抗压强度低于钛合金,这就限制了其在颌骨骨折特别是承重骨折中的应用[48]。下颌骨骨折是颌面部最常见的骨折类型,因有强大的升颌肌群和降颌肌群附着,常导致骨折块移位,咬合错乱,因此,下颌骨骨折的固定物要求有一定的机械性能。镁及镁基材料在颌面部骨折中的应用[49-53],见表3。ORASSI等[51]通过有限元分析发现,镁合金钢板及螺钉在治疗4种下颌骨骨折类型中的固定性能表现与钛合金基本相同。ANSOMS等[52]研究显示,镁合金固定材料具有足够的机械性能承担下颌角骨折部分所承担的咀嚼力。随后有学者利用有限元分析模型模拟在正颌外科手术中使用WE43镁合金螺钉固定下颌升支矢状劈开截骨的骨折线,结果显示在咀嚼负载情况下WE43镁合金仍能稳定固定骨折线,打开了生物可降解镁合金在正颌外科方面应用的大门,提供了更多的可能性和研究方向[53-54]。"

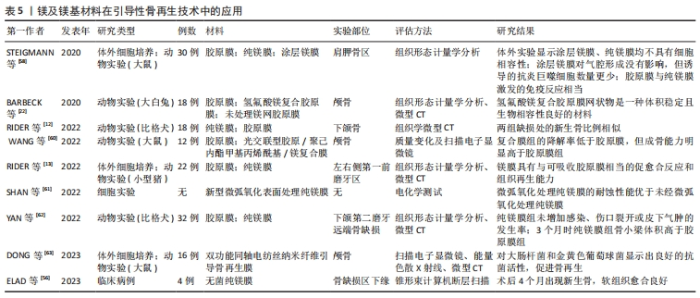
2.3.2 镁及镁基材料在口腔骨再生领域中的应用 引导性骨再生技术:引导性骨再生技术中使用的引导性骨再生生物膜作为硬组织和软组织之间的屏障,可以防止软组织干扰成骨,从而为成骨细胞的分化提供足够空间,进而诱导成骨,治疗因牙周炎、拔牙、肿瘤或囊肿等疾病导致的骨缺损。临床使用的生物膜一类是可吸收的,例如合成聚合物聚乳酸-羟基乙酸、聚乙烯亚胺、聚(L-乳酸)或天然聚合物(胶原蛋白,壳聚糖)等;另一类是不可吸收的,例如钛网或聚四氟乙烯等[56]。目前临床广泛使用的胶原蛋白类可吸收生物膜虽具有良好的生物相容性与可降解性,但机械性能不足,在修复大面积及垂直性骨缺损时可能会塌陷,导致缺损部位成骨作用不足,而不可吸收类的钛网虽具有足够的机械性能,但可能需二次手术取出,应用也受到限制。多项体内外研究表明,采用热轧纯镁(镁95.5%)生物膜具有与胶原蛋白膜相当的成骨能力,并且具有良好的机械强度与缓慢降解特性,可以维持骨形成的空间,达到治疗骨高度不足及大面积骨缺损的目的[12-13]。FROSECCHI[57]将纯镁生物膜与胶原蛋白膜联合应用于治疗临床中牙槽骨的复杂骨缺损,取得了良好的成骨效果。也有学者采用不同表面处理方法提升了镁膜的免疫性及成骨性[57-61],在降低镁膜降解率的同时也降低了骨组织细菌感染和骨吸收风险(表5)。"


骨替代材料:通常同种骨、异种骨、异体骨及合成骨替代材料被用作移植材料用于颅颌面骨缺损的治疗,随着材料在医学领域的飞速发展,更多的合成材料被引入作为骨移植材料,包括钙磷陶瓷、生物玻璃及以聚乳酸为基础的可吸收聚合物。其中,钙磷陶瓷由于与骨组织的矿物组成和性质最接近而最受青睐[64],主要以颗粒状羟基磷灰石、β-磷酸三钙盐或两者的混合物应用于临床治疗,如上颌窦底提升及拔牙后的位点保存技术[65]。但有研究证实颗粒状的磷酸钙材料也存在风险,比如可能由于其尖锐的颗粒状边缘引起上颌窦底黏膜穿孔,或者作为骨替代材料吸收率不佳,不能完全被骨组织所替代。与传统磷酸钙材料相比,镁基材料可以在中性pH值下发生化学反应产生鸟粪石(MgNH4PO4×?6H2O)等矿物,此类矿物在pH值7.01-9.62范围内具有比磷酸钙更好的吸收率,并且释放的镁离子可刺激骨骼重塑,展示出更好的溶解性和生物相容性[66]。同时,以磷酸镁为基础的镁基骨替代物大多是通过矿物粉末与水溶液混合胶凝反应加工得来,有学者通过在表面活性剂稳定的亲油性液体中乳化不同剂量比的钙镁磷水泥浆体,成功合成了具有理想尺寸分布的完全球形无创颗粒,进一步加速了镁基材料成为一种更理想骨替代材料的可能[67]。 2.3.3 镁及镁基材料在牙种植体表面涂层的应用 钛合金是牙种植体修复中应用最广泛的一种金属种植体,它可以为后期的全冠修复提供稳定基础。然而,由于纯钛种植体表面缺乏抗菌活性,种植体植入后为口腔内各种革兰阳性和阴性细菌的生长和入侵创造了良好环境,增加了感染风险,导致种植体松动[68]。因此,对钛种植体表面进行改性提高其抗菌活性、促进骨细胞附着,已成为目前解决上述问题的重要途径[69]。镁离子作为体内的重要元素参与骨愈合,通过调节体内L型钙通道降低细胞内钙浓度,可抑制种植体植入后活性氧的产生,以此减少炎症因子释放,进而发挥抗炎和抗氧化作用[70];此外,镁离子可以随尿排泄,通过肾小管重吸收,具有良好的体内生物降解性。有体外研究结果表明,镁基涂层钛植入物可增强骨髓间充质干细胞的增殖、增加成骨标志物(碱性磷酸酶、骨钙素、骨桥蛋白、骨唾液蛋白、Runx2)的表达、增加Ⅰ型胶原沉积和抗菌活性,同时,镁离子通过调节血清钙、磷水平预防局部高钙、高磷,从而避免局部矿化,更好地促进骨愈合[71-73]。有学者将通过半粉末冶金技术将石墨烯纳米片和碳纳米管纳米系统作为增强体均匀分散在镁锌合金涂层的钛种植体中,可显著降低种植体外周的炎症反应和氧化应激,更好地抑制口腔中革兰阳性菌的生长,促进人牙龈成纤维细胞的增殖活性和成骨细胞的分化潜能,从而提高种植体的骨整合稳定性[74]。 2.3.4 镁及镁基材料在颌面部、口腔内软组织再生及牙组织工程中的应用 多项研究表明,镁及镁基材料在颌面部及口腔内软组织再生领域亦有良好的应用前景。镁对人成纤维细胞的迁移、黏附和口腔黏膜再生有积极作用。镁对成纤维细胞活性的影响可能对种植体表面改性和促进种植体颈部周围软组织愈合有良好影响。有研究表明,镁基金属植入物在降解过程中会释放出可溶的镁离子和氢气,具有神经保护和抗氧化作用[75],中间产物镁石榴石B可通过磷脂酰肌醇3-激酶-蛋白激酶B信号转导促进神经干细胞分化、刺激局部神经再生、改善微循环,这与镁离子促进成骨的信号通路相吻。镁对神经疼痛的治疗有一定效果,补充镁对颌面部周围神经损伤的恢复也有效果,但镁在运动神经中的应用有待进一步研究。在牙组织工程方面,镁离子可诱导人牙髓细胞的增殖、分化和矿化,参与牙髓修复过程,在盖髓剂中引入镁离子被认为是促进牙组织再生的新途径[76-78]。同时,镁对牙体硬组织的矿化也十分重要,WON等[79]利用单细胞RT-PCR方法检测镁离子渗透性离子通道TRPM7和镁离子转运体CNNM4在大鼠成牙本质细胞中的表达,发现这2种分子可能是成牙本质细胞中主要的镁离子调节剂,CNNM4可能选择性参与根尖牙本质的形成或矿化。 2.3.5 镁及镁基材料作为药物负载材料的应用 目前,颌面部术后均有发生感染的风险,全身抗生素应用仍然是临床中最常用的预防及治疗方法,但该方法在感染部位可能无法达到足够的药物浓度。目前学者们通过实验使用可降解镁基材料制作了负载抗菌药物的局部给药系统,促进成骨及骨修复的同时成功克服了植入物周围血药浓度低的问题。BAKHSHESHI-RAD等[80]用新型空间保持器技术制备了负载强力霉素的镁-钙-TiO2复合支架,药物释放曲线显示出初始突释和持续释放2种模式,表现出明显的抑菌活性。也有学者发现,负载不同浓度四环素的镁锌合金支架可有效对抗金黄色葡萄球菌和大肠杆菌的活性,并且四环素浓度为1%-5%的镁锌支架能够更有效实现药物缓释,保证抗菌效果的长效性[81]。目前与镁基材料有关的药物负载局部给药系统的研究多集中于体外研究,鲜有有应用于体内的报道。 综上所述,镁基材料在颌面部及口内软组织再生、牙组织工程及药物负载中的应用大部分仍停留在体外实验阶段,仍需大量临床前期实验逐步向临床转化。目前的大多数研究将现有生物材料与镁基材料对比,未来可尝试将现有及临床使用成熟的生物材料与镁基材料联用,进一步拓展对镁基材料的应用。 2.4 生物安全性 目前,国内外对可降解金属植入物的生物学评价体系尚不完善。镁合金植入机体后降解太快,不能保持足够的稳定性,手术第1周内会出现机体不良反应,如局部肿胀和疼痛。因此,设计出耐腐蚀、生物相容性更稳定的镁基合金仍是一个亟待解决的问题[82-83]。等离子体电解氧化又称微弧氧化,是在传统阳极氧化基础上发展起来的一种环保的表面处理方法,可以在镁合金表面形成致密、厚实的陶瓷层。利用微弧氧化技术对镁基合金表面进行修饰,可提高合金的初始耐腐蚀性和机械强度,保护基材免受周围生物流体的伤害。另外,加入其他合金元素如稀土元素等可有效改善镁合金的力学性能、耐腐蚀性能和生物性能,但稀土元素不是人体必需的微量元素,过量可能会引起细胞毒性。对于不可降解金属的标准细胞毒性测试程序,即将金属放入培养液中一段时间,然后在该培养液中培养靶细胞以确定细胞毒性[84]。但这一标准不适用于可降解金属,因为它们会在培养基中发生降解,这是一个矛盾点。因此,建立并完善新的可降解金属体内外生物评价体系变得尤为重要。"

| [1] VUJOVIĆ S, DESNICA J, STANIŠIĆ D, et al. Applications of biodegradable magnesium-based materials in reconstructive oral and maxillofacial surgery: a review. Molecules. 2022;27(17):5529. [2] XING F, LI S, YIN D, et al. Recent progress in Mg-based alloys as a novel bioabsorbable biomaterials for orthopedic applications. J Magnes Alloy. 2022; 10(6):1428-1456. [3] LI Y, WANG J, YUE J, et al. High magnesium prevents matrix vesicle‐mediated mineralization in human bone marrow‐derived mesenchymal stem cells via mitochondrial pathway and autophagy. Cell Biol Int. 2018;42(2):205-215. [4] HUSE EC. A New Ligature. Chic Med J Exam. 1878;37(2):171-172. [5] WITTE F. The history of biodegradable magnesium implants: a review. Acta Biomater. 2010;6(5):1680-1692. [6] MCBRIDE E. Magnesium screw and nail transfixion in fractures. South Med J. 1938;31:508-514. [7] EGGEBRECHT H, RODERMANN J, HUNOLD P, et al. Images in cardiovascular medicine. Novel magnetic resonance-compatible coronary stent: the absorbable magnesium-alloy stent. Circulation. 2005;112(18):e303-304. [8] ROJAEE R, FATHI M, RAEISSI K. Controlling the degradation rate of AZ91 magnesium alloy via sol-gel derived nanostructured hydroxyapatite coating. Mater Sci Eng C Mater Biol Appl. 2013;33(7):3817-3825. [9] SCHALLER B, SAULACIC N, BECK S, et al. In vivo degradation of a new concept of magnesium-based rivet-screws in the minipig mandibular bone. Mater Sci Eng C Mater Biol Appl. 2016;69:247-254. [10] YU Y, JIN G, XUE Y, et al. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017;49:590-603. [11] FUCHS A, KRECZY D, BRÜCKNER T, et al. Bone regeneration capacity of newly developed spherical magnesium phosphate cement granules. Clin Oral Investig. 2022;26(3):2619-2633. [12] RIDER P, KAČAREVIĆ Ž P, ELAD A, et al. Biodegradable magnesium barrier membrane used for guided bone regeneration in dental surgery. Bioact Mater. 2022;14:152-168. [13] RIDER P, KAČAREVIĆ ŽP, ELAD A, et al. Analysis of a pure magnesium membrane degradation process and its functionality when used in a guided bone regeneration model in beagle dogs. Materials. 2022;15(9):3106. [14] ZHAO Z, YU W, YANG W, et al. Dual-Protection Inorganic-Protein Coating on Mg-Based Biomaterials through Tooth-Enamel-Inspired Biomineralization. Adv Mater. 2024;36(21):e2313211. [15] BYUN SH, LIM HK, CHEON KH, et al. Biodegradable magnesium alloy (WE43) in bone-fixation plate and screw. J Biomed Mater Res B Appl Biomater. 2020; 108(6):2505-2512. [16] YANG GF, KIM YC, HAN HS, et al. In vitro dynamic degradation behavior of new magnesium alloy for orthopedic applications. J Biomed Mater Res B Appl Biomater. 2015;103(4):807-815. [17] AMUKARIMI S, MOZAFARI M. Biodegradable magnesium biomaterials—Road to the clinic. Bioengineering. 2022;9(3):107. [18] GE J, YANG C, WANG Y, et al. Comparison of different grafting materials for treatment of bone defect distal to the molar in canine. Clin Implant Dent Relat Res. 2018;20(4):444-454. [19] TSAO YT, SHIH YY, LIU YA, et al. Knockdown of SLC41A1 magnesium transporter promotes mineralization and attenuates magnesium inhibition during osteogenesis of mesenchymal stromal cells. Stem Cell Res Ther. 2017;8:1-10. [20] TORRONI A, XIANG C, WITEK L, et al. Biocompatibility and degradation properties of WE43 Mg alloys with and without heat treatment: In vivo evaluation and comparison in a cranial bone sheep model. J Craniomaxillofac Surg. 2017;45(12): 2075-2083. [21] WANG J, XU J, LIU W, et al. Biodegradable magnesium (Mg) implantation does not impose related metabolic disorders in rats with chronic renal failure. Sci Rep. 2016;6(1):26341. [22] BARBECK M, KÜHNEL L, WITTE F, et al. Degradation, bone regeneration and tissue response of an innovative volume stable magnesium-supported GBR/GTR barrier membrane. Int J Mol Sci. 2020;21(9):3098. [23] LIU Y, WANG DL, HUANG YC, et al. Hydrogen inhibits the osteoclastogenesis of mouse bone marrow mononuclear cells. Mater Sci Eng C Mater Biol Appl. 2020;110:110640. [24] MRAIED H, WANG W, CAI W. Influence of chemical heterogeneity and microstructure on the corrosion resistance of biodegradable WE43 magnesium alloys. J Mater Chem B. 2019;7(41):6399-6411. [25] PROBST FA, FLIEFEL R, BURIAN E, et al. Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate-poly (D, L-lactide-co-glycolide) scaffolds. Sci Rep. 2020;10(1):2062. [26] JÄHN K, SAITO H, TAIPALEENMÄKI H, et al. Intramedullary Mg2Ag nails augment callus formation during fracture healing in mice. Acta Biomater. 2016;36:350-360. [27] DÍAZ-TOCADOS JM, HERENCIA C, MARTÍNEZ-MORENO JM, et al. Magnesium chloride promotes osteogenesis through Notch signaling activation and expansion of mesenchymal stem cells. Sci Rep. 2017;7(1):7839. [28] HUNG CC, CHAYA A, LIU K, et al. The role of magnesium ions in bone regeneration involves the canonical Wnt signaling pathway. Acta Biomater. 2019;98:246-255. [29] WANG Z, LIU Q, LIU C, et al. Mg2+ in β‐TCP/Mg–Zn composite enhances the differentiation of human bone marrow stromal cells into osteoblasts through MAPK‐regulated Runx2/Osx. J Cell Physiol. 2020;235(6):5182-5191. [30] GAO P, FAN B, YU X, et al. Biofunctional magnesium coated Ti6Al4V scaffold enhances osteogenesis and angiogenesis in vitro and in vivo for orthopedic application. Bioact Mater. 2020;5(3):680-693. [31] CECCHINATO F, KARLSSON J, FERRONI L, et al. Osteogenic potential of human adipose-derived stromal cells on 3-dimensional mesoporous TiO2 coating with magnesium impregnation. Mater Sci Eng C Mater Biol Appl. 2015;52:225-234. [32] EL-RASHIDY AA, ROETHER JA, HARHAUS L, et al. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017;62:1-28. [33] BYAMBAA B, ANNABI N, YUE K, et al. Bioprinted osteogenic and vasculogenic patterns for engineering 3D bone tissue. Adv Healthc Mater. 2017;6(16):1700015. [34] PERCIVAL CJ, RICHTSMEIER JT. Angiogenesis and intramembranous osteogenesis. Dev Dyn. 2013;242(8):909-922. [35] DIRCKX N, VAN HUL M, MAES C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Res C Embryo Today. 2013;99(3):170-191. [36] GU Y, ZHANG J, ZHANG X, et al. Three-dimensional printed Mg-doped β-TCP bone tissue engineering scaffolds: effects of magnesium ion concentration on osteogenesis and angiogenesis in vitro. Tissue Eng Regen Med. 2019;16:415-429. [37] MAU JR, HAWKINS KM, WOO SL, et al. Design of a new magnesium-based anterior cruciate ligament interference screw using finite element analysis. J Orthop Translat. 2020;20:25-30. [38] SADOWSKA JM, GINEBRA MP. Inflammation and biomaterials: role of the immune response in bone regeneration by inorganic scaffolds. J Mater Chem B. 2020;8(41):9404-9427. [39] RAHMATI M, STÖTZEL S, KHASSAWNA TE, et al. Early osteoimmunomodulatory effects of magnesium–calcium–zinc alloys. J Tissue Eng. 2021;12: 20417314211047100. [40] GLOBIG P, WILLUMEIT-RÖMER R, MARTINI F, et al. Optimizing an osteosarcoma-fibroblast coculture model to study antitumoral activity of magnesium-based biomaterials. Int J Mol Sci. 2020;21(14):5099. [41] MARUKAWA E, TAMAI M, TAKAHASHI Y, et al. Comparison of magnesium alloys and poly‐l‐lactide screws as degradable implants in a canine fracture model. J Biomed Mater Res B Appl Biomater. 2016;104(7):1282-1289. [42] BONITHON R, KAO AP, FERNÁNDEZ MP, et al. Multi-scale mechanical and morphological characterisation of sintered porous magnesium-based scaffolds for bone regeneration in critical-sized defects. Acta Biomater. 2021;127:338-352. [43] WITTE F, KAESE V, HAFERKAMP H, et al. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials. 2005;26(17):3557-3563. [44] NAUJOKAT H, RUFF CB, KLÜTER T, et al. Influence of surface modifications on the degradation of standard-sized magnesium plates and healing of mandibular osteotomies in miniature pigs. Int J Oral Maxillofac Surg. 2020;49(2):272-283. [45] SCHALLER B, BURKHARD JPM, CHAGNON M, et al. Fracture healing and bone remodeling with human standard-sized magnesium versus polylactide–co-glycolide plate and screw systems using a mini-swine craniomaxillofacial osteotomy fixation model. J Oral Maxillofac Surg. 2018;76(10):2138-2150. [46] LEONHARDT H, ZIEGLER A, LAUER G, et al. Osteosynthesis of the mandibular condyle with magnesium-based biodegradable headless compression screws show good clinical results during a 1-year follow-up period. J Oral Maxillofac Surg. 2021;79(3):637-643. [47] KOZAKIEWICZ M, GABRYELCZAK I. Bone union quality after fracture fixation of mandibular head with compression magnesium screws. Materials. 2022;15(6): 2230. [48] MAO L, SHEN L, CHEN J, et al. Enhanced bioactivity of Mg–Nd–Zn–Zr alloy achieved with nanoscale MgF2 surface for vascular stent application. ACS Appl Mater Interfaces. 2015;7(9):5320-5330. [49] IMWINKELRIED T, BECK S, SCHALLER B. Pre-clinical testing of human size magnesium implants in miniature pigs: implant degradation and bone fracture healing at multiple implantation sites. Mater Sci Eng C Mater Biol Appl. 2020;108: 110389. [50] KOZAKIEWICZ M. Change in pull-out force during resorption of magnesium compression screws for osteosynthesis of mandibular condylar fractures. Materials. 2021;14(2):237. [51] ORASSI V, FISCHER H, DUDA GN, et al. In silico biomechanical evaluation of WE43 magnesium plates for mandibular fracture fixation. Front Bioeng Biotechnol. 2022;9:803103. [52] ANSOMS P, BARZEGARI M, VANDER SLOTEN J, et al. Coupling biomechanical models of implants with biodegradation models: A case study for biodegradable mandibular bone fixation plates. J Mech Behav Biomed Mater. 2023;147: 106120. [53] LEE JH, HAN HS, KIM YC, et al. Stability of biodegradable metal (Mg-Ca-Zn alloy) screws compared with absorbable polymer and titanium screws for sagittal split ramus osteotomy of the mandible using the finite element analysis model. J Craniomaxillofac Surg. 2017;45(10):1639-1646. [54] CHEN K, LU Y, TANG H, et al. Effect of strain on degradation behaviors of WE43, Fe and Zn wires. Acta Biomater. 2020;113:627-645. [55] KOZAKIEWICZ M, GABRYELCZAK I, BIELECKI-KOWALSKI B. Clinical evaluation of magnesium alloy osteosynthesis in the mandibular head. Materials. 2022; 15(3):711. [56] ELAD A, RIDER P, ROGGE S, et al. Application of biodegradable magnesium membrane shield technique for immediate dentoalveolar bone regeneration. Biomedicines. 2023;11(3):744. [57] FROSECCHI M. Horizontal and Vertical Defect Management with a Novel Degradable Pure Magnesium Guided Bone Regeneration (GBR) Membrane—A Clinical Case. Medicina. 2023;59(11):2009. [58] STEIGMANN L, JUNG O, KIEFERLE W, et al. Biocompatibility and immune response of a newly developed volume-stable magnesium-based barrier membrane in combination with a PVD coating for guided bone regeneration (GBR). Biomedicines. 2020;8(12):636. [59] KANNO T, SUKEGAWA S, FURUKI Y, et al. Overview of innovative advances in bioresorbable plate systems for oral and maxillofacial surgery. Jpn Dent Sci Rev. 2018;54(3):127-138. [60] WANG F, XIA D, WANG S, et al. Photocrosslinkable Col/PCL/Mg composite membrane providing spatiotemporal maintenance and positive osteogenetic effects during guided bone regeneration. Bioact Mater. 2022;13:53-63. [61] SHAN X, XU Y, KOLAWOLE SK, et al. Degradable pure magnesium used as a barrier film for oral bone regeneration. J Funct Biomater. 2022;13(4):298. [62] YAN ZY, ZHU JH, LIU GQ, et al. Feasibility and Efficacy of a Degradable Magnesium‐Alloy GBR Membrane for Bone Augmentation in a Distal Bone‐Defect Model in Beagle Dogs. Bioinorg Chem Appl. 2022;2022(1):4941635. [63] DONG Y, YAO L, CAI L, et al. Antimicrobial and pro-osteogenic coaxially electrospun magnesium oxide nanoparticles-polycaprolactone/parathyroid hormone-polycaprolactone composite barrier membrane for guided bone regeneration. Int J Nanomedicine. 2023;18:369-383. [64] FUCHS A, KRECZY D, BRÜCKNER T, et al. Bone regeneration capacity of newly developed spherical magnesium phosphate cement granules. Clin Oral Investig. 2022;26(3):2619-2633. [65] NABIYOUNI M, BRÜCKNER T, ZHOU H, et al. Magnesium-based bioceramics in orthopedic applications. Acta Biomater. 2018;66:23-43. [66] WANG N, MA Y, SHI H, et al. Mg-, Zn-, and Fe-based alloys with antibacterial properties as orthopedic implant materials. Front Bioeng Biotechnol. 2022;10:888084. [67] BLUM C, BRÜCKNER T, EWALD A, et al. Mg: Ca ratio as regulating factor for osteoclastic in vitro resorption of struvite biocements. Mater Sci Eng C Mater Biol Appl. 2017;73:111-119. [68] HERNÁNDEZ-ESCOBAR D, PAJARES-CHAMORRO N, CHATZISTAVROU X, et al. Tailored Coatings for Enhanced Performance of Zinc–Magnesium Alloys in Absorbable Implants. ACS Biomater Sci Eng. 2023;10(1):338-354. [69] BAI Y, WANG L, ZHAO L, et al. Antibacterial and Antioxidant Effects of Magnesium Alloy on Titanium Dental Implants. Comput Math Methods Med. 2022;2022(1):6537676. [70] WON S, HUH Y H, CHO LR, et al. Cellular response of human bone marrow derived mesenchymal stem cells to titanium surfaces implanted with calcium and magnesium ions. Tissue Eng Regen Med. 2017;14:123-131. [71] MIHAILESCU N, STAN GE, DUTA L, et al. Structural, compositional, mechanical characterization and biological assessment of bovine-derived hydroxyapatite coatings reinforced with MgF2 or MgO for implants functionalization. Mater Sci Eng C Mater Biol Appl. 2016;59:863-874. [72] ONDER S, CALIKOGLU-KOYUNCU AC, KAZMANLI K, et al. Magnesium doping on TiN coatings affects mesenchymal stem cell differentiation and proliferation positively in a dose-dependent manner. Biomed Mater Eng. 2018;29(4):427-438. [73] TAO ZS, ZHOU WS, HE XW, et al. A comparative study of zinc, magnesium, strontium-incorporated hydroxyapatite-coated titanium implants for osseointegration of osteopenic rats. Mater Sci Eng C Mater Biol Appl. 2016;62:226-232. [74] LIU JR, WANG XY, SABERI A, et al. The effect of Co-encapsulated GNPs-CNTs nanofillers on mechanical properties, degradation and antibacterial behavior of Mg-based composite. J Mech Behav Biomed Mater. 2023;138:105601. [75] ZHANG J, ZHANG B, ZHANG J, et al. Magnesium promotes the regeneration of the peripheral nerve. Front Cell Dev Biol. 2021;9:717854. [76] KONG YY, HU XL, ZHONG YQ, et al. Magnesium-enriched microenvironment promotes odontogenic differentiation in human dental pulp stem cells by activating ERK/BMP2/Smads signaling. Stem Cell Res Ther. 2019;10(1):378. [77] ZHENG JM, KONG YY, LI YY, et al. MagT1 regulated the odontogenic differentiation of BMMSCs induced byTGC-CM via ERK signaling pathway. Stem Cell Res Ther. 2019;10(1):48. [78] SALEM RM, ZHANG C, CHOU LS. Effect of magnesium on dentinogenesis of human dental pulp cells. Int J Biomater. 2021;2021:6567455. [79] WON J, KIM JH, OH SB. Molecular expression of Mg2+ regulator TRPM7 and CNNM4 in rat odontoblasts. Arch Oral Biol. 2018;96:182-188. [80] BAKHSHESHI-RAD HR, HAMZAH E, STAIGER MP, et al. Drug release, cytocompatibility, bioactivity, and antibacterial activity of doxycycline loaded Mg-Ca-TiO2 composite scaffold. Mater Des. 2018;139:212-221. [81] DAYAGHI E, BAKHSHESHI-RAD HR, HAMZAH E, et al. Magnesium-zinc scaffold loaded with tetracycline for tissue engineering application: in vitro cell biology and antibacterial activity assessment. Mater Sci Eng C Mater Biol Appl. 2019;102:53-65. [82] KIM SR, LEE KM, KIM JH, et al. Biocompatibility evaluation of peo-treated magnesium alloy implants placed in rabbit femur condyle notches and paravertebral muscles. Biomater Res. 2022;26(1):29. [83] ZHAO Z, YU W, YANG W, et al. Dual‐Protection Inorganic‐Protein Coating on Mg-Based Biomaterials through Tooth-Ename-Inspired Biomineralization. Adv Mater. 2024;36(21):e2313211. [84] KLÍMA K, ULMANN D, BARTOŠ M, et al. A complex evaluation of the in-vivo biocompatibility and degradation of an extruded ZnMgSr absorbable alloy implanted into rabbit bones for 360 days. Int J Mol Sci. 2021;22(24):13444. |
| [1] | Yang Xuetao, Zhu Menghan, Zhang Chenxi, Sun Yimin, Ye Ling. Applications and limitations of antioxidant nanomaterials in oral cavity [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2044-2053. |
| [2] | Xu Chang, Jiang Mingzhu, Liu Xin, Yan Weijun. Three-dimensional finite element analysis of implant anchorage combined with torque auxiliary arch to lower anterior teeth [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1971-1978. |
| [3] | Wu Yanting, Li Yu, Liao Jinfeng. Magnesium oxide nanoparticles regulate osteogenesis- and angiogenesis-related gene expressions to promote bone defect healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1885-1895. |
| [4] | Kang Zirui, Wu Yang, Song Hailong, Yang Qiaoyun, Zang Lixiang, Xu Dongliang. Finite element analysis of implants with different crown-to-implant ratios under different bone conditions [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 319-328. |
| [5] | Zhang Qiya, Tong Yixiang, Yang Shijiao, Zhang Yumeng, Deng Ling, Wu Wei, Xie Yao, Liao Jian, Mao Ling. In vitro biocompatibility of graded glass infiltrated ultra-translucent zirconia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 443-450. |
| [6] | Tan Fengyi, Xie Jiamin, Pan Zhenfeng, Zhang Xinxu, Zheng Zetai, Zeng Zhiying, Zhou Yanfang. Effect and mechanism of collagen combined with microneedles in treatment of skin photoaging [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 451-458. |
| [7] | Wang Zhuo, Sun Panpan, Cheng Huanzhi, Cao Tingting. Application of chitosan in repair and regeneration of oral hard and soft tissues [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 459-468. |
| [8] | Liu Yu, Gong Senyi, Yang Lihua, Li Weifeng, Hu Yuwen, Yan Qinbiao, Guo Meijin. Isolation, identification, and application of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 194-203. |
| [9] | Zhao Shuai, Li Dongyao, Wei Suiyan, Cao Yijing, Xu Yan, Xu Guoqiang. Biocompatibility of poly(vinylidene fluoride) piezoelectric bionic periosteum prepared by electrospinning [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 730-737. |
| [10] | Liu Lu, Zhong Chang, Yu Xin, Ren Chenyuan, Gong Yangyang, Zhou Ping, Wang Yingbin. Academic progress and clinical application of in vitro synthetic microenvironment to promote maturation of human pluripotent stem cell-derived cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7856-7862. |
| [11] | Ma Yucong, Ouyang Zhengzheng, Liu Xiaojie, Yang Sifei. Tracking of research trends and hotspots in medical magnesium alloy materials [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7470-7480. |
| [12] | Wang Jingshuai, Zhang Xiaotong, Zhang Yange, Wan Zedong, Kong Lingwei, Cao Haiying, Jin Yu. Comparison of Mg-Li-Gd alloy and stainless steel intramedullary nail for fixation of femoral annular hemi-defects in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7261-7268. |
| [13] | Wu Ziwei, Luo Yicai, Wei Yinge, Liao Hongbing. Application of poly(lactic-co-glycolic acid) copolymer in stomatology [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7393-7404. |
| [14] | Wang Simin, Zhang Dezhou, Zhao Jing, Wang Chaoqun, Li Kun, Chen Jie, Bai Xue, Zhao Hailong, Zhang Shaojie, Ma Yuan, Hao Yunteng, Yang Yang, Li Zhijun, Shi Jun, Wang Xing. Artificial intelligence and cervical spine image recognition: application prospects and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(33): 7231-7240. |
| [15] | Shi Chunrong, He Jiaxu, Deng Lishan, Wang Hailan, Zhao Aimin, Yu Yiling, Geng Haixia, Song Weijun. Application of graphene oxide in field of oral implant restoration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 6118-6126. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||