Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (34): 7415-7422.doi: 10.12307/2025.883
Previous Articles Next Articles
Induction of M1/M2 polarization of macrophages by lipopolysaccharides and titanium particles in peri-implant tissues
Deng Ran1, Wei Yi1, Ji Xiaowei2, 3
- 1Department of Dentistry and Endodontics, 2Department of Prosthodontics and Implantology, First Affiliated Hospital of Xinjiang Medical University (Affiliated Stomatological Hospital), Urumqi 830054, Xinjiang Uygur Autonomous Region, China; 3Institute of Stomatology of Xinjiang Uygur Autonomous Region, Urumqi 830054, Xinjiang Uygur Autonomous Region, China
-
Received:2024-08-23Accepted:2024-10-16Online:2025-12-08Published:2025-01-17 -
Contact:Ji Xiaowei, Associate chief physician, Doctoral candidate, Department of Prosthodontics and Implantology, First Affiliated Hospital of Xinjiang Medical University (Affiliated Stomatological Hospital), Urumqi 830054, Xinjiang Uygur Autonomous Region, China; Institute of Stomatology of Xinjiang Uygur Autonomous Region, Urumqi 830054, Xinjiang Uygur Autonomous Region, China -
About author:Deng Ran, Master candidate, Department of Dentistry and Endodontics, First Affiliated Hospital of Xinjiang Medical University (Affiliated Stomatological Hospital), Urumqi 830054, Xinjiang Uygur Autonomous Region, China -
Supported by:Oral Health Promotion and Oral Medicine Development Western Clinical Research Fund of Chinese Stomatological Association, No. CSA-2022-01 (to JXW)
CLC Number:
Cite this article
Deng Ran, Wei Yi, Ji Xiaowei. Induction of M1/M2 polarization of macrophages by lipopolysaccharides and titanium particles in peri-implant tissues[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7415-7422.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
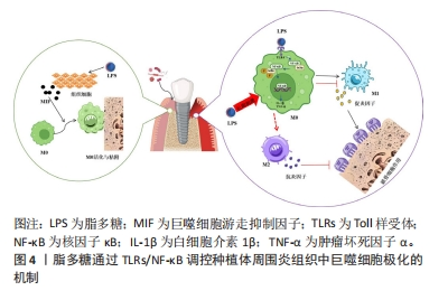
2.1 引起种植体周围组织巨噬细胞极化的相关病因 种植体周围炎的病因复杂多样,包括菌斑微生物、粘接剂残留、种植体不密合等医源性因素,以及糖尿病、牙周病史和吸烟等危险因素[8],与其相关的致病机制主要是细菌引发的炎症免疫反应和特异性、非特异性的免疫防御机制,其中细菌的毒力分子脂多糖和钛颗粒可能通过诱导植体周围组织中巨噬细胞极化来参与相关免疫反应。 2.1.1 脂多糖 细菌是众多口腔炎性疾病的始动因素,脂多糖作为细菌的主要毒力因子,可引起种植体周围炎。脂多糖是革兰阴性杆菌细胞壁特有的糖脂复合物,能有效激活人体的免疫系统,可破坏植体周围骨免疫平衡,引起骨组织破坏。脂多糖可刺激巨噬细胞产生白细胞介素8、白细胞介素6等多种促炎因子,使其显著向M1极化[9]。脂多糖还可诱导钛种植体周围骨髓间质细胞炎症中巨噬细胞游走抑制因子的产生,参与宿主对微生物感染时发生的系列应激反应,进而活化巨噬细胞,抑制其游走移动,增强其黏附、吞噬作用[10]。 脂多糖通过多个信号通路对种植体周围组织中巨噬细胞的极化发挥调节作用,如图4所示,脂多糖能激活核因子κB信号通路,上调肿瘤坏死因子α、白细胞介素1β、白细胞介素1等促炎因子的表达,进而诱导巨噬细胞向M1表型极化。此外,诱导 M1的极化过程中,还存在细胞外调节蛋白激酶/丝裂原活化蛋白激酶(extracellular regulated protein kinases/mitogen-activated protein kinase,ERK/MAPK)信号通路的磷酸化[11]。牙周致病菌也可通过脂多糖刺激巨噬细胞表面的白细胞分化抗原14(cluster of differentiation 14,CD14)、Toll样受体和NOD样受体诱导巨噬细胞分化为M1型[12]。病原菌的脂多糖不仅能与传统Toll样受体(尤其是Toll样受体4)结合,还能与细胞内模式识别受体半胱氨酸天冬氨酸蛋白酶11(cysteinyl aspartate specific proteinase,caspase-11)结合,从而进一步触发炎症小体,导致大量白细胞介素1β和白细胞介素18的释放,使种植体周围炎性细胞聚集和炎症信号放大,利于M1型巨噬细胞极化[13]。"

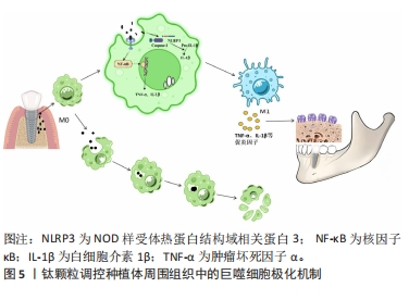
然而,有趣的是巨噬细胞长期暴露于脂多糖时会倾向于一种耐受的低反应状态,产生促炎递质的能力下降,但参与免疫抑制和伤口愈合的介质表达升高,这反而有利于M2极化[14],这种免疫耐受反应可能与巨噬细胞中的线粒体甘油3-磷酸脱氢酶(mitochondrial glycerol 3-phosphate dehydrogenase,GPD2)有关。在被脂多糖激活的巨噬细胞中,GPD2促进葡萄糖氧化和乙酰辅酶a的产生,使组蛋白乙酰化,进而诱导编码炎症递质的基因,驱动炎症反应[14]。GPD2结合病原菌刺激和葡萄糖氧化进程,可解释脂多糖短期暴露时的炎症诱导和长期暴露时的炎症抑制,从而将脂多糖作用时间和炎症进展的平衡联系起来[15]。 2.1.2 钛颗粒 大量证据表明植入物失效与植入物周围的无菌性炎症(碎屑、钛离子和微粒脱落)也有关联[16],骨科植入物的无菌性松动也归因于钛碎片和钛颗粒[17]。种植手术中和植体结构松动产生的摩擦力能使钛颗粒释放,口腔致病菌发酵糖类物质形成的酸性环境可以破坏TiO2层,产生钛颗粒[18]。如图5所示,钛颗粒作为异物颗粒被释放到周围组织中,可以引起多种免疫反应,打破植体周围形成的异物平衡状态,进而导致种植体周围炎。"

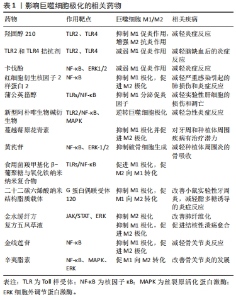
Hippo信号是成骨细胞在成骨过程中分化、形成和稳态的重要级联途径[19]。ZHU等[20]研究发现钛颗粒可以通过抑制Yes相关蛋白磷酸化进而抑制小鼠胚胎成骨细胞中Hippo/YAP信号通路的激活,最终抑制其成骨分化的生物学行为。钛颗粒可以通过调节种植体周围牙龈组织中淋巴细胞和巨噬细胞的极化,进一步引起成骨细胞-破骨细胞活性的不平衡,最终导致口腔种植体骨结合的失败[21]。人体种植体周围炎组织中也发现了大量巨噬细胞和淋巴细胞浸润[18],其中巨噬细胞可以吞噬钛离子并释放白细胞介素1β和肿瘤坏死因子α等促炎因子,这些细胞因子可以通过核因子κB受体激活因子配体(receptor activator of nuclear factor κB ligand,RANKL)/核因子κB受体激活因子(receptor activator of nuclear factor κB,RANK)/骨保护素信号通路激活破骨细胞,从而促进骨吸收和种植体周围疾病的发展[22]。在种植体周围炎患者植周组织中钛颗粒可引起RANKL、白细胞介素33表达升高[23],并且发现M1/M2巨噬细胞比例增加[24],这也证实了上述观点。相关研究发现miRNA let-7f-5p可通过靶向白细胞介素10诱导M1巨噬细胞极化,从而促进磨损颗粒诱导的骨溶解[25]。这些证据都表明钛颗粒可能参与调控种植体周围组织中巨噬细胞的极化过程。 钛颗粒引起周围组织中巨噬细胞极化还与其形态和数目有关。由碱性蚀刻处理产生的二氧化钛纳米刺突对巨噬细胞胞体的物理刺激可以激活炎症小体,在吞噬过程中,刺突状颗粒在细胞膜上施加机械应力,导致K+流出和炎症小体以胱冬肽酶1和NOD样受体热蛋白结构域相关蛋白3(NOD-like receptor thermal protein domain associated protein 3,NLRP3)的方式激活[26],进而调节巨噬细胞向M1型的极化,但纳米棒和粗颗粒则没有这类作用。相关研究表明巨噬细胞对TiO2颗粒的吞噬可呈大小和剂量依赖性[27]。种植体周围软组织成分中金属微量元素诱导巨噬细胞活化的作用呈现剂量依赖性的趋势[28]。金属颗粒还可在组织中慢慢积聚引起相关免疫炎症反应,高浓度的纳米金属颗粒是骨组织和口腔黏膜组织慢性免疫病理炎症的主要诱因,在组织中的积累达到临界剂量后可凝固到微米大小,导致先天免疫系统细胞的早期死亡[28]。 2.2 脂多糖和钛颗粒引起种植体周围组织M1/M2极化的相关通路 2.2.1 Toll样受体通路 Toll样受体主要表达于吞噬细胞和抗原提呈细胞,可在M2型巨噬细胞中高度表达,从而在促进组织修复和伤口愈合中发挥抗炎和血管生成作用[29],还可识别脂多糖、肽聚糖、脂蛋白等具有病原相关分子模式的分子。Toll样受体识别到脂多糖后,下游的核因子κB、MAPK和磷脂酰肌醇-3-激酶/丝苏氨酸激酶等信号通路可被激活[30],产生大量促炎因子(白细胞介素1β、白细胞介素6和肿瘤坏死因子α)等,从而调控巨噬细胞的M1/M2极化方向,其中Toll样受体家族中的Toll样受体2和Toll样受体4通常被认为是调节种植体周围炎的关键免疫识别受体[13],与种植体周围组织中的巨噬细胞极化密切相关。Toll样受体还是免疫反应中的模式识别受体,通过调节免疫B细胞浸润、RANKL/骨保护素和炎症因子等表达差异和比例介导种植体周围炎中的骨丢失[31]。 (1)脂多糖/Toll样受体4:Toll样受体4可通过受体二聚作用结合并接触下游的MAPKs、核因子kB和蛋白酪氨酸激酶2/信号传导转录激活因子1[32],激活其相关信号通路的组分使促炎因子表达增加来调节炎症[33],使巨噬细胞发生M1型极化。还能识别脂多糖并驱动炎性细胞因子的分泌,其中瞬时受体电位7介导了脂多糖诱导巨噬细胞激活所必需的胞质Ca2+升高,在Toll样受体4内吞作用和激活核因子κB中发挥重要作用[34]。相关研究表明多功能毒力因子TcpC可以下调细胞外调节蛋白酪氨酸激酶和p38/核因子κB信号通路,上调蛋白酪氨酸激酶2/信号传导转录激活因子6信号通路,抑制M1而促进M2巨噬细胞极化,从而有利于尿致病性大肠杆菌逃离巨噬细胞介导的炎症抗感染反应[35],这也与TcpC能阻碍Toll样受体4信号通路有关。具有牙周炎易感性的患者在种植治疗后牙龈组织中的Toll样受体4表达水平升高[36]。植入物无菌性松动也与Toll样受体激活有关,Toll样受体可识别脂多糖等病原相关分子模式,产生慢性炎症,最终加速破骨细胞的形成和增殖[37]。这都表明脂多糖/Toll样受体4可能参与调控种植体周围炎组织中巨噬细胞极化过程。 (2)钛颗粒与Toll样受体:吞噬相关受体如Toll样受体2和Toll样受体4,能识别含有病原相关分子模式的钛颗粒,长时间暴露在这种环境中会引起钛颗粒介导的种植体周围骨溶解[38]。在磨损颗粒与Toll样受体介导炎症的相关研究中发现,聚乙烯颗粒可与组织细胞中的Toll样受体2结合进而激活相关炎症信号通路,金属离子也被证明与Toll样受体4、M1极化呈正相关[39]。 钛颗粒作为异物颗粒进入周围组织后可触发Toll样受体2、Toll样受体4的表达,激活巨噬细胞的吞噬作用,而吞噬作用增强脂多糖诱导的核因子 κB激活[40],可诱导M1巨噬细胞的极化。相关研究发现TiO2纳米刺突与巨噬细胞接触后可能会触发吞噬相关受体的表达,如Toll样受体2、Toll样受体4等,并最终激活吞噬作用及促炎递质的产生,利于M1的极化[41]。其他异物颗粒也具有类似作用,硅酸钙颗粒对小鼠巨噬细胞没有毒性,但可以通过Toll样受体2介导的核因子κB和c-Jun氨基末端蛋白激酶通路诱导炎症反应,增加促炎因子肿瘤坏死因子α的表达[42]。氧化铁纳米颗粒也可通过激活Toll样受体4信号通路促进巨噬细胞自噬和炎症反应[43]。但在炎症缓解阶段Toll样受体2的诱导可以参与到抗炎反应中,促进抗炎因子的表达,而这些变化则利于M2的极化[42]。 2.2.2 核因子κB通路 核因子κB作为在Toll样受体下游的信号通路之一广泛参与机体的炎症反应和免疫应答的过程,Toll样受体/核因子κB信号通路是介导炎性免疫反应的重要信号通路[44],在许多免疫性疾病中发挥重要作用。在M1巨噬细胞分化过程中,核因子κB的活化在炎症基因表达中起关键作用[45],种植体周围炎症的发生发展也与其密切相关。当脂多糖与相关受体结合后,可激活核因子κB抑制因子α(Inhibitor of nuclear factor-KBa,IkBa)激酶,使IkBa磷酸化,进而使核因子κB从核因子κB/IkBa复合物中释放出来,并活化、暴露核定位域,形成二聚体,迅速发生核转位,从而启动靶基因表达如肿瘤坏死因子α和白细胞介素1等[46],使种植体周围组织中的微环境发生改变,诱导巨噬细胞向促炎M1型极化。在体外,脂多糖、干扰素γ 和白细胞介素26处理人或小鼠巨噬细胞均可促进M1巨噬细胞极化,进一步的机制研究发现受白细胞介素26刺激的M1样巨噬细胞内核因子κB和信号转导与转录激活因子1(signal transducerand activator of transcription 1, STAT1)等信号通路被激活[47]。受体活化C激酶1高表达的口腔鳞癌细胞可以通过其核因子κB信号通路抑制人单核细胞的迁移,促进M2样巨噬细胞的极化[48]。 2.2.3 其他相关通路 除了激活Toll样受体及下游核因子κB、MAPK和STAT等信号通路,还可通过许多信号通路对种植体周围组织中巨噬细胞M1/M2极化发挥调控作用。细胞免疫球蛋白黏蛋白3(T-cell immunoglobulin and mucin domain-containing protein 3,Tim-3)与配体半乳糖凝集素9(Galectin-9,Gal-9)特异性结合后可通过调节巨噬细胞功能在感染中发挥重要作用,脂多糖刺激早期上调Tim-3/Gal-9相互作用,激活Tim-3信号通路进而抑制M1极化,然而在后期则下调相互作用,抑制Tim-3信号通路进而促进M1极化,抑制M2极化[49]。Wnt信号蛋白/β-连环蛋白信号通路可通过增加Toll样受体的表达,在激活巨噬细胞向M1极化的过程中发挥重要作用[50]。基质细胞衍生因子1/趋化因子(C-X-C基元)受体4作为一个机械力敏感的信号轴,也可通过招募外周血中循环炎性单核细胞和促进巨噬细胞M1极化来增加牙周组织中M1/M2比率,从而介导正畸牙根外吸收[51]。在牙周炎中, 影响Notch信号通路可调节巨噬细胞极化类型,使M1/M2比例发生变化,进而影响牙周炎进展[32]。 2.3 相关药物调控Toll样受体/核因子κB通路影响巨噬细胞极化的研究进展 相关药物可通过靶向作用于Toll样受体和核因子κB分子,抑制Toll样受体和核因子κB的激活或信号传导,减少促炎因子的分泌,可以达到治疗相关疾病的目的[44]。目前一些药物可通过作用于Toll样受体改变巨噬细胞极化方向,进而影响炎症进展,如羟固醇210是一种半合成氧甾醇,可通过抑制Toll样受体2和Toll样受体4信号传导进而调节巨噬细胞极化,使巨噬细胞发挥抗炎作用[52]。在局灶性脑梗死的大鼠模型中减弱Toll样受体2和Toll样受体4的诱导可减轻脑缺血后的炎症反应,也能促进神经功能恢复[53]。部分药物也能将核因子κB作为干预靶点影响巨噬细胞极化来治疗相关炎性疾病,如一项体外研究中发现卡伐酚对脂多糖诱导的核因子κB核易位和活化有抑制作用,可通过抑制ERK1/2和核因子κB的细胞机制发挥相关的抗炎作用[54]。红细胞衍生核因子2样蛋白2是一种核转录因子,可通过调节核因子κB介导的巨噬细胞极化,抑制M1极化并促进M2极化,在严重感染引起的肺损伤和炎症中发挥保护作用[55]。还有一些药物对二者都有干预作用,如蒲公英甾醇可抑制Toll样受体/核因子κB通路进而减少肿瘤坏死因子α、白细胞介素6、白细胞介素1β、干扰素γ和白细胞介素4的释放,减轻刀豆蛋白A诱导的肝组织病理学损伤和细胞凋亡[56]。新型阿朴啡生物碱衍生物可作为有效的Toll样受体2拮抗剂,阻断核因子kB和MAPK信号通路的激活,逆转巨噬细胞极化和中性粒细胞浸润,进而治疗急性炎症[57]。 在以骨丢失为主的口腔炎性疾病中,部分药物在通过影响巨噬细胞极化减轻炎症反应方面取得进展。蔓越莓原花青素在牙周和种植体周围疾病治疗中发挥抗炎和调控巨噬细胞极化作用,当受脂多糖刺激的巨噬细胞暴露于蔓越莓原花青素时,M1极化显著降低,M2极化显著增加[9]。黄芪苷具有良好的抗炎和抗氧化作用,可通过抑制核因子κB的激活和细胞外信号相关激酶1/2的磷酸化,下调RANKL的表达水平,从而抑制破骨细胞生成,显著减轻种植体周围炎的骨吸收[58]。 如表1所示,目前靶向巨噬细胞极化的相关药物研究更多的集中于药物递送和中医药作用机制研究方面,如食用菌羧甲基化β-葡聚糖与氧化铁纳米颗粒结合形成纳米复合物在体外研究中可通过活化的Toll样受体-核因子κB通路将M2样骨髓源性巨噬细胞转化为 M1表型,对诱导M1型巨噬细胞进行免疫治疗有协同作用[59]。二十二碳六烯酸纳米结构脂质载体可通过抑制巨噬细胞M1型极化并促进其重定位至M2型从而减轻脂多糖诱导的炎症,并在小鼠实验性牙周炎中发挥骨保护作用[60]。在中医药研究方面,在金水缓纤方[61]、复方五凤草液等中药方剂研究中发现了巨噬细胞极化的参与[62],许多中药有效成分作用机制也与其相关,如金线莲苷和辛夷脂素均可下调P65/核因子κB信号通路[11,63],抑制M1极化和促进M2极化,从而减轻骨关节炎症状。但至今相关药物仍集中于作用机制的基础研究中,进一步的临床转化还需进行更多的实验和探索。"

| [1] 郑桂婷,徐燕,吴明月.种植体周围疾病治疗的专家共识及治疗方法的进展[J].国际口腔医学杂志,2020,47(6):725-731. [2] WU X, QIAO S, WANG W, et al. Melatonin prevents periimplantitis via suppression of TLR4/NF-κB. Acta Biomater. 2021;134:325-336. [3] DREYER H, GRISCHKE J, TIEDE C, et al. Epidemiology and risk factors of peri‐implantitis: A systematic review. J Periodontal Res. 2018;53(5):657-681. [4] 毛舜,谢辉.种植体周围病治疗新进展[J].中国口腔种植学杂志,2020, 25(2):85. [5] DARBY I. Risk factors for periodontitis & peri‐implantitis. Periodontol 2000. 2022;90(1):9-12. [6] WANG X, LI Y, FENG Y, et al. The role of macrophages in osseointegration of dental implants: An experimental study in vivo. J Biomed Mater Res A. 2020;108(11):2206-2216. [7] SHAPOURI‐MOGHADDAM A, MOHAMMADIAN S, VAZINI H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425-6440. [8] 杨成雪,喻正文,范芹.种植体周围病的病因及危险因素[J].临床口腔医学杂志,2020,36(5):313-316. [9] GALARRAGA‐VINUEZA ME, DOHLE E, RAMANAUSKAITE A, et al. Anti‐inflammatory and macrophage polarization effects of Cranberry Proanthocyanidins (PACs) for periodontal and peri‐implant disease therapy. J Periodontal Res. 2020;55(6):821-829. [10] 夏冬景,裴浩.脂多糖诱导钛种植体周围骨髓间质细胞炎症中的巨噬细胞游走抑制因子[J].中国组织工程研究,2015,19(32):5123. [11] LU J, ZHANG H, PAN J, et al. Fargesin ameliorates osteoarthritis via macrophage reprogramming by downregulating MAPK and NF-κB pathways. Arthritis Res Ther. 2021;23(1):142. [12] SUN X, GAO J, MENG X, et al. Polarized macrophages in periodontitis: characteristics, function, and molecular signaling. Front Immunol. 2021;12: 763334. [13] JIAO P, LI Z, LI B, et al. The role of caspase-11 and pyroptosis in the regulation of inflammation in peri-implantitis. J Inflamm Res. 2023:16:4471-4479. [14] HOPPSTÄDTER J, DEMBEK A, LINNENBERGER R, et al. Toll-like receptor 2 release by macrophages: an anti-inflammatory program induced by glucocorticoids and lipopolysaccharide. Front Immunol. 2019;10:1634. [15] LANGSTON P K, NAMBU A, JUNG J, et al. Glycerol phosphate shuttle enzyme GPD2 regulates macrophage inflammatory responses. Nat Immunol. 2019; 20(9):1186-1195. [16] BOSSHARDT DD, CHAPPUIS V, BUSER D. Osseointegration of titanium, titanium alloy and zirconia dental implants: current knowledge and open questions. Periodontol 2000. 2017;73(1):22-40. [17] FRETWURST T, NELSON K, TARNOW DP, et al. Is metal particle release associated with peri-implant bone destruction? An emerging concept. J Dent Res. 2018;97(3):259-265. [18] SHAH R, PENMETSA DSL, THOMAS R, et al. Titanium Corrosion: Implications For Dental Implants. Eur J Prosthodont Restor Dent. 2016;24(4):171-180. [19] BYUN MR, KIM AR, HWANG JH, et al. FGF2 stimulates osteogenic differentiation through ERK induced TAZ expression. Bone. 2014;58:72-80. [20] ZHU W, MING P, QIU J, et al. Effect of titanium ions on the Hippo/YAP signaling pathway in regulating biological behaviors of MC3T3‐E1 osteoblasts. J Appl Toxicol. 2018;38(6):824-833. [21] PARK JB, JEON Y, KO Y. Effects of titanium brush on machined and sand‐blasted/acid‐etched titanium disc using confocal microscopy and contact profilometry. Clin Oral Implan Res. 2015;26(2):130-136. [22] EGER M, STERER N, LIRON T, et al. Scaling of titanium implants entrains inflammation-induced osteolysis. Sci Rep. 2017;7(1):39612. [23] BERRYMAN Z, BRIDGER L, HUSSAINI HM, et al. Titanium particles: An emerging risk factor for peri-implant bone los]. Saudi Dent J. 2020;32(6):283-292. [24] GALARRAGA VINUEZA ME, OBREJA K, BEGIC A, et al. Macrophage polarization in peri‐implantitis lesions. Clin Oral Implan Res. 2020;31:6-6. [25] GAO X, GE J, LI W, et al. NF-κB/let-7f-5p/IL-10 pathway involves in wear particle-induced osteolysis by inducing M1 macrophage polarization. Cell Cycle. 2018;17(17):2134-2145. [26] WANG J, CHEN H J, HANG T, et al. Physical activation of innate immunity by spiky particles. Nat Nanotechnol. 2018;13(11):1078-1086. [27] RAO AJ, GIBON E, MA T, et al. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomater. 2012;8(7):2815-2823. [28] LABIS V, BAZIKYAN E, SIZOVA S, et al. Immunopathological Inflammation in the Evolution of Mucositis and Peri-Implantitis. Int J Mol Sci. 2022;23(24): 15797. [29] WU J, ZHANG L, SHI J, et al. Macrophage phenotypic switch orchestrates the inflammation and repair/regeneration following acute pancreatitis injury. EBioMedicine. 2020:58:102920. [30] VERGADI E, IERONYMAKI E, LYRONI K, et al. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. 2017;198(3): 1006-1014. [31] 韦雨杏,董皓,韦惠平,等.种植体周围炎炎性组织差异表达基因的筛选及验证[J].中国组织工程研究,2023,27(30):4844. [32] 苑舒月,刘春艳,刘冰,等.巨噬细胞的极化与牙周炎[J].中国组织工程研究,2023,27(17):2699. [33] LAI J, LIU Y, LIU C, et al. Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB and MAPK signaling pathways. Inflammation. 2017;40:1-12. [34] SCHAPPE MS, SZTEYN K, STREMSKA ME, et al. Chanzyme TRPM7 mediates the Ca2+ influx essential for lipopolysaccharide-induced toll-like receptor 4 endocytosis and macrophage activation. Immunity. 2018;48(1):59-74.e5. [35] FANG J, OU Q, WU B, et al. TcpC inhibits M1 but promotes M2 macrophage polarization via regulation of the MAPK/NF-κB and Akt/STAT6 pathways in urinary tract infection. Cells. 2022;11(17):2674. [36] KOUTOUZIS T, CATANIA D, NEIVA K, et al. Innate immune receptor expression in peri‐implant tissues of patients with different susceptibility to periodontal diseases. J Periodontol. 2013;84(2):221-229. [37] ZHANG Y, XIAO J, DENG S, et al. IRAK-4 in macrophages contributes to inflammatory osteolysis of wear particles around loosened hip implants. Innate Immun. 2021;27(6):470-482. [38] CHANMEE T, ONTONG P, KONNO K, et al. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6(3):1670-1690. [39] 张晓非,吕震,王小泉,等.人工关节假体周围无菌性松动的发生机制[J].天津医药,2020,48(6):572-576. [40] UENO T, YAMAMOTO Y, KAWASAKI K. Phagocytosis of microparticles increases responsiveness of macrophage-like cell lines U937 and THP-1 to bacterial lipopolysaccharide and lipopeptide. Sci Rep. 2021;11(1):6782. [41] KARTIKASARI N, YAMADA M, WATANABE J, et al. Titania nanospikes activate macrophage phagocytosis by ligand-independent contact stimulation. Sci Rep. 2022;12(1):12250. [42] LAI S, CHEN L, CAO W, et al. Dicalcium silicate induced proinflammatory responses through TLR2-mediated NF-κB and JNK pathways in the murine RAW 264.7 macrophage cell line. Mediat Inflamm. 2018;2018:8167932. [43] JIN R, LIU L, ZHU W, et al. Iron oxide nanoparticles promote macrophage autophagy and inflammatory response through activation of toll-like Receptor-4 signaling. Biomaterials. 2019;203:23-30. [44] 李依峣,鲁元,王旭,等.Toll样受体家族在神经炎症性疾病中的作用机制研究进展[J].中国新药杂志,2022,31(16):1602-1607. [45] WANG N, LIANG H, ZEN K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front Immunol. 2014;5:614. [46] 余元勋.中国分子白血病学[M].合肥:安徽科学技术出版社,2016. [47] LIN YH, WANG YH, PENG YJ, et al. Interleukin 26 skews macrophage polarization towards M1 phenotype by activating cJUN and the NF-κB pathway. Cells-Basel. 2020;9(4):938. [48] DAN H, LIU S, LIU J, et al. RACK1 promotes cancer progression by increasing the M2/M1 macrophage ratio via the NF‐κB pathway in oral squamous cell carcinoma. Mol Oncol. 2020;14(4):795-807. [49] ZHANG W, ZHANG Y, HE Y, et al. Lipopolysaccharide mediates time-dependent macrophage M1/M2 polarization through the Tim-3/Galectin-9 signalling pathway. Exp Cell Res. 2019;376(2):124-132. [50] AVERY D, MORANDINI L, SHEAKLEY LS, et al. Canonical Wnt signaling enhances pro-inflammatory response to titanium by macrophages. Biomaterials. 2022;289:121797. [51] FANG XY, ZHAN YX, ZHOU XM, et al. CXCL12/CXCR4 mediates orthodontic root resorption via regulating the M1/M2 ratio. J Dent Res. 2022;101(5): 569-579. [52] WANG F, STAPPENBECK F, TANG LY, et al. Oxy210, a semi-synthetic oxysterol, exerts anti-inflammatory effects in macrophages via inhibition of toll-like receptor (TLR) 4 and TLR2 signaling and modulation of macrophage polarization. Int J Mol Sci. 2022;23(10):5478. [53] NALAMOLU KR, CHALLA SR, FORNAL CA, et al. Attenuation of the induction of TLRs 2 and 4 mitigates inflammation and promotes neurological recovery after focal cerebral ischemia. Transl Stroke Res. 2021;12:923-936. [54] SOMENSI N, RABELO TK, GUIMARÃES AG, et al. Carvacrol suppresses LPS-induced pro-inflammatory activation in RAW 264.7 macrophages through ERK1/2 and NF-kB pathway. Int Immunopharmacol. 2019;75:105743. [55] LUO J, WANG J, ZHANG J, et al. Nrf2 deficiency exacerbated CLP-induced pulmonary injury and inflammation through autophagy-and NF-κB/PPARγ-mediated macrophage polarization. Cells. 2022;11(23):3927. [56] SANG R, YU Y, GE B, et al. Taraxasterol from Taraxacum prevents concanavalin A-induced acute hepatic injury in mice via modulating TLRs/NF-κB and Bax/Bc1-2 signalling pathways. Artif Cell Nanomed B. 2019;47(1):3929-3937. [57] YANG J, PAN Y, ZENG X, et al. Discovery of novel aporphine alkaloid derivative as potent TLR2 antagonist reversing macrophage polarization and neutrophil infiltration against acute inflammation. Acta Pharm Sin B. 2023;13(9):3782-3801. [58] WANG X, CHEN X, ZHANG Z, et al. Asperuloside Prevents Peri-Implantitis via Suppression of NF-κB and ERK1/2 on Rats. Pharmaceuticals-Base. 2022;15(8):1027. [59] ZHANG J, TYLER HL, HARON MH, et al. Macrophage activation by edible mushrooms is due to the collaborative interaction of toll-like receptor agonists and dectin-1b activating beta glucans derived from colonizing microorganisms. Food Funct. 2019;10(12):8208-8217. [60] 何捷.二十二碳六烯酸纳米结构脂质载体调控巨噬细胞极化治疗牙周炎的研究[D].长春:吉林大学,2024. [61] 刘览,邵栋,殷晓红,等.金水缓纤方抑制JAK/STAT和ERK信号阻抑巨噬细胞M2极化改善肺纤维化的机制[J].中华中医药杂志,2023,38(5):1967-1973. [62] 钱佳燕.复方五凤草液调控M1/M2巨噬细胞极化干预结核性溃疡的临床研究[D].南京:南京中医药大学,2020. [63] ZHOU F, MEI J, HAN X, et al. Kinsenoside attenuates osteoarthritis by repolarizing macrophages through inactivating NF-κB/MAPK signaling and protecting chondrocytes. Acta Pharm Sin B. 2019;9(5):973-985. [64] YUNNA C, MENGRU H, LEI W, et al. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. [65] 周宇翔,沈烈军,万诗雨,等.骨免疫调节特性骨组织工程支架在修复骨缺损中的应用和发展[J].中国组织工程研究,2024,28(29):4734. [66] 张照欣,王新革,葛泽阳,等.钛种植体愈合期牙龈炎性浸润及巨噬细胞极化的研究[J].实用口腔医学杂志,2022,38(3):294-299. [67] CARCUAC O, BERGLUNDH T. Composition of human peri-implantitis and periodontitis lesions. J Dent Res. 2014;93(11):1083-1088. [68] 张照欣.巨噬细胞在种植体软组织界面分布特征及介导病理性骨吸收作用的实验研究[D].西安:中国人民解放军空军军医大学,2022. [69] ALBREKTSSON T, TENGVALL P, AMENGUAL L, et al. Osteoimmune regulation underlies oral implant osseointegration and its perturbation. Front Immunol. 2023;13:1056914. [70] 席向东,陈德胜,杨超,等.MMP-9、TNF-α、IL-1 在无菌性松动界膜组织中的表达及相关性研究[J].生物骨科材料与临床研究,2021,18(1):5-8,12. [71] CHANG Y, JIANG K, ZHANG L, et al. Application of next-generation sequencing technology in the detection of pathogenic bacteria of the periprosthetic joint infection after arthroplasty. Int Wound J. 2023;20(6):2121-2128. [72] 王健,崔玉宝,李杭,等.金属磨损颗粒诱导假体无菌性松动的研究进展[J].东南大学学报(医学版),2024,43(3):483-486. [73] SHI Y, ZHANG Q, BI H, et al. Decoding the multicellular ecosystem of vena caval tumor thrombus in clear cell renal cell carcinoma by single-cell RNA sequencing. Genome Biol. 2022;23(1):87. [74] LIN P, JI HH, LI YJ, et al. Macrophage plasticity and atherosclerosis therapy. Front Mol Biosci. 2021;8:679797. |
| [1] | Chen Haojie, Wang Dai, Shen Shan. Immune inflammatory microenvironment mechanisms in peri-implantitis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2054-2062. |
| [2] | Wen Guangwei, Zhen Yinghao, Zheng Taikeng, Zhou Shuyi, Mo Guoye, Zhou Tengpeng, Li Haishan, Lai Yiyi. Effects and mechanisms of isoginkgetin on osteoclastogenesis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1348-1358. |
| [3] | Pan Hongfei, Zhuang Zhenbing, Xu Baiyun, Yang Zhangyang, Lin Kairui, Zhan Bingqing, Lan Jinghan, Gao Heng, Zhang Nanbo, Lin Jiayu. Inhibitory effects of different concentrations of auranofin on M1 macrophage function and its therapeutic potential in diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1390-1397. |
| [4] | You Huijuan, Wu Shuzhen, Rong Rong, Chen Liyuan, Zhao Yuqing, Wang Qinglu, Ou Xiaowei, Yang Fengying. Macrophage autophagy in lung diseases: two-sided effects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1516-1526. |
| [5] | Fu Zhenyi, Li Junhao, Zhang Yating, He Yunkai, Liu Junyu, Wei Yunhao, Liu Jiaxin. Schwann cells promote peripheral nerve regeneration: retrospect and prospect [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1236-1246. |
| [6] | Wang Jie, Huang Rui, Zhang Ye, Shou Zhaoxi, Yao Jie, Liu Chenxi, Liao Jian. Role and mechanism of probiotics in peri-implantitis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 901-907. |
| [7] | Cao Wenqi, Feng Xiuzhi, Zhao Yi, Wang Zhimin, Chen Yiran, Yang Xiao, Ren Yanling. Effect of macrophage polarization on osteogenesis-angiogenesis coupling in type 2 diabetic osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 917-925. |
| [8] | Yang Hu, Zheng Yu, Jia Chengming, Wang Tong, Zhang Guangfei, Ji Yaoyao. Immune microenvironment regulates bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 701-710. |
| [9] | Xie Peisen, Guan Zhenpeng, Wei Xianjie, Zhang Keshi, Kang Qingyuan, Xiao Wentao, Guo Xiaoshuai. Cerium dioxide nanoparticles regulate expression of inflammatory factors in M1 macrophages and affect fibroblast co-culture system [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 375-383. |
| [10] | Sun Huiwen, Guo Qiangqiang, Wang Wei, Wu Jie, Xi Kun, Gu Yong. Engineered stem cell bionic periosteum coordinates immune inflammation and vascularization to promote bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 21-33. |
| [11] | Wang Wentao, Hou Zhenyang, Wang Yijun, Xu Yaozeng. Apelin-13 alleviates systemic inflammatory bone loss by inhibiting macrophage M1 polarization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1548-1555. |
| [12] | Huang Ting, Zheng Xiaohan, Zhong Yuanji, Wei Yanzhao, Wei Xufang, Cao Xudong, Feng Xiaoli, Zhao Zhenqiang. Effects of macrophage migration inhibitory factor on survival, proliferation, and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1380-1387. |
| [13] | Chang Jinxia, Liu Yufei, Niu Shaohui, Wang Chang, Cao Jianchun. Visualization analysis of macrophage polarization in tissue repair process [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1486-1496. |
| [14] | Chi Wenxin, Zhang Cunxin, Gao Kai, Lyu Chaoliang, Zhang Kefeng. Mechanism by which nobiletin inhibits inflammatory response of BV2 microglia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1321-1327. |
| [15] | Yu Ting, Lyu Dongmei, Deng Hao, Sun Tao, Cheng Qian. Icariin pretreatment enhances effect of human periodontal stem cells on M1-type macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1328-1335. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

