Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (1): 21-33.doi: 10.12307/2025.551
Previous Articles Next Articles
Engineered stem cell bionic periosteum coordinates immune inflammation and vascularization to promote bone regeneration
Sun Huiwen, Guo Qiangqiang, Wang Wei, Wu Jie, Xi Kun, Gu Yong
- First Affiliated Hospital of Soochow University, Suzhou 215000, Jiangsu Province, China
-
Received:2024-07-18Accepted:2024-09-05Online:2026-01-08Published:2025-06-13 -
Contact:Gu Yong, MD, Associate chief physician, Master’s supervisor, First Affiliated Hospital of Soochow University, Suzhou 215000, Jiangsu Province, China -
About author:Sun Huiwen, First Affiliated Hospital of Soochow University, Suzhou 215000, Jiangsu Province, China Guo Qiangqiang, Master candidate, First Affiliated Hospital of Soochow University, Suzhou 215000, Jiangsu Province, China. Sun Huiwen and Guo Qiangqiang contributed equally to this article. -
Supported by:National Natural Science Foundation of China, No. 82072438, 82272501 (to GY); National Natural Science Foundation of China, No. 82102589 (to XK)
CLC Number:
Cite this article
Sun Huiwen, Guo Qiangqiang, Wang Wei, Wu Jie, Xi Kun, Gu Yong. Engineered stem cell bionic periosteum coordinates immune inflammation and vascularization to promote bone regeneration[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 21-33.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
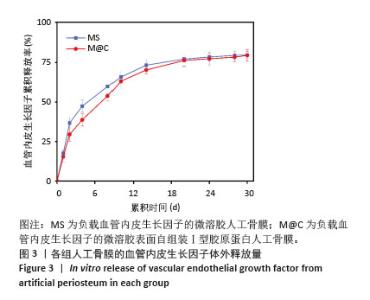
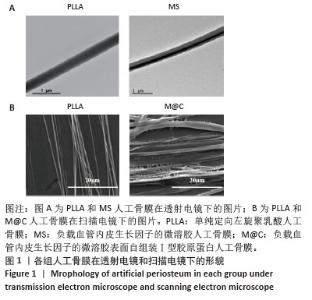
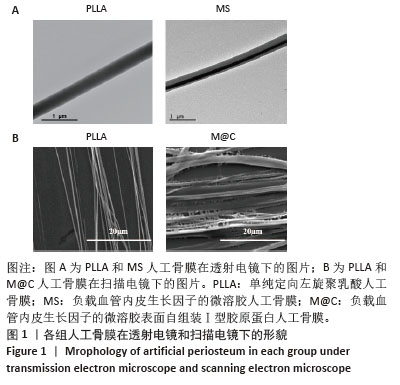
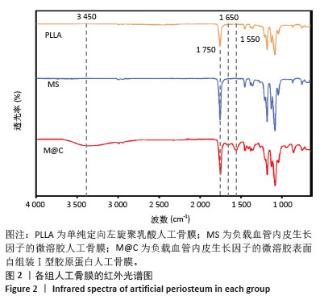
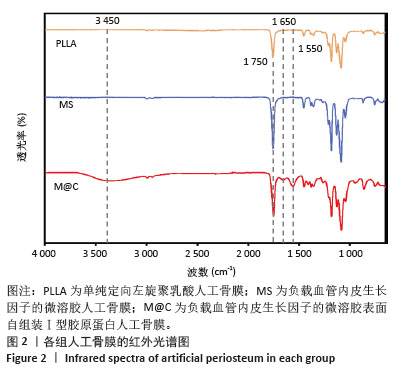
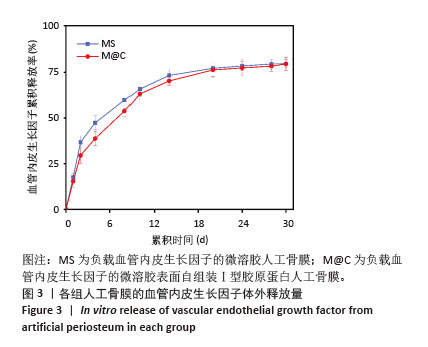
2.3 人工骨膜中血管内皮生长因子的体外缓释 用ELISA试剂盒检测血管内皮生长因子的体外释放量。如图3所示,MS组和M@C组在前2 d 的血管内皮生长因子释放速度较快,总释放量分别为(36.4±1.5)%和(30.1±1.4)%,随后释放速度逐渐减缓,不过释放率都具有持续性,其中M@C组的释放速度比MS组更为缓慢,至第14天的总释放率分别为(68.6±1.1)%和(71.8±0.7)%,最终释放时间达30 d时,累计释放率分别为(78.3±0.4)%和(77.9±0.3)%,M@C组的释放速度更加缓慢,释放总量也较少,这可能归因于外层Ⅰ型胶原蛋白的存在,直接影响了人工骨膜内部生物活性分子的释放动力学。"

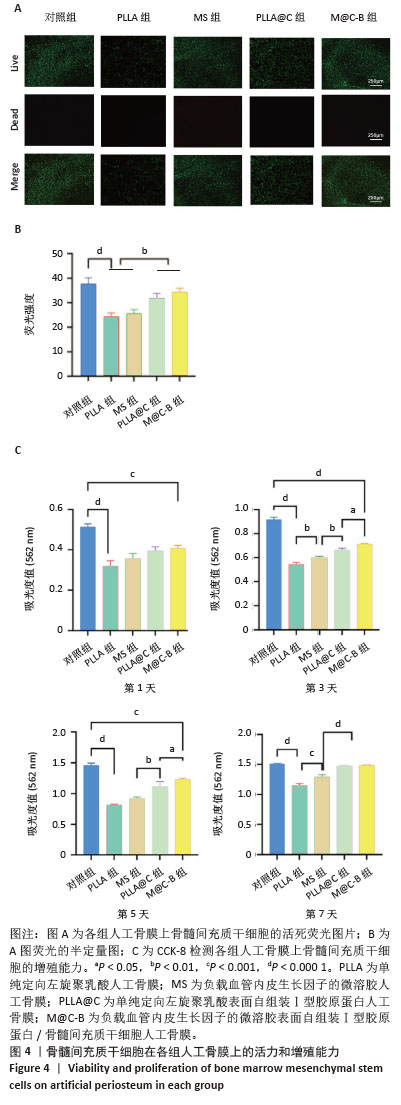
2.4 人工骨膜的生物相容性 如图4A活死染色显示,细胞接种在不同人工骨膜和普通培养板上进行培养,由于PLLA和MS表面疏水性质,细胞黏附性较低,在其上生长的活细胞数量明显少于Ⅰ型胶原蛋白自组装组(PLLA@C和M@C-B),并且死细胞数量也较多,可能是由于添加的透明质酸都在纤维内部,对PLLA的疏水性没有改善。而胶原自组装后,由于胶原的加入显著提升了材料的生物相容性,图4B为活死染色的荧光强度。细胞增殖实验结果如图4C所示,骨髓间充质干细胞能在各材料表面增殖生长,第1-7天各组细胞数目总体是上升趋势,对照组细胞增殖速率优于各材料组,到第7天时,对照组与PLLA@C组、M@C-B组无明显统计学差异。总之,由于普通培养板专业的细胞培养性能,材料组的细胞增殖速率均低于对照组,但是胶原的加入显著增强了材料的生物相容性。 "
2.5 人工骨膜的体外成骨能力 图5为各组人工骨膜的成骨分化指标,碱性磷酸酶是成骨分化早期的指标,可反映出人工骨膜对骨髓间充质干细胞早期成骨分化的影响。图5A可见各组人工骨膜的成骨分化均为上升趋势,其中PLLA组和对照组染色较浅,密度也较低,而MS组、PLLA@C组和M@C-B组与前两组相比,染色和密度均有所提高,其中PLLA@C组和M@C-B组提高较明显。骨桥蛋白为成骨分化的中晚期指标,图5B荧光图片显示,骨桥蛋白的表达与碱性磷酸酶类似。钙结节是成骨分化的晚期标识物,经茜素红染色后为铁锈色,染色结果显示PLLA@C组和M@C-B组钙结节面积最大,吸光度最强,说明了Ⅰ型胶原蛋白的加入不仅通过其表面丰富的细胞膜配体增强骨髓间充质干细胞的生物学效应,而且在一定程度上能够促进其向成骨分化。图5C-E的半定量结果与上述结果一致。 2.6 人工骨膜的体外成血管能力 恢复骨膜的血液供应是骨修复的关键过程,如图6A所示,在体外的成血管实验中对照组和PLLA组的成血管能力没有显著差异,而负载了血管内皮生长因子的MS组和M@C-B组的成血管能力有明显提高,其血管长度更长,呈节段状分布,血管染色呈网状形态,并且交叉节点明显多于对照组和PLLA组。对管状节点数量和管状结构长度进行计数,见图6B。 "
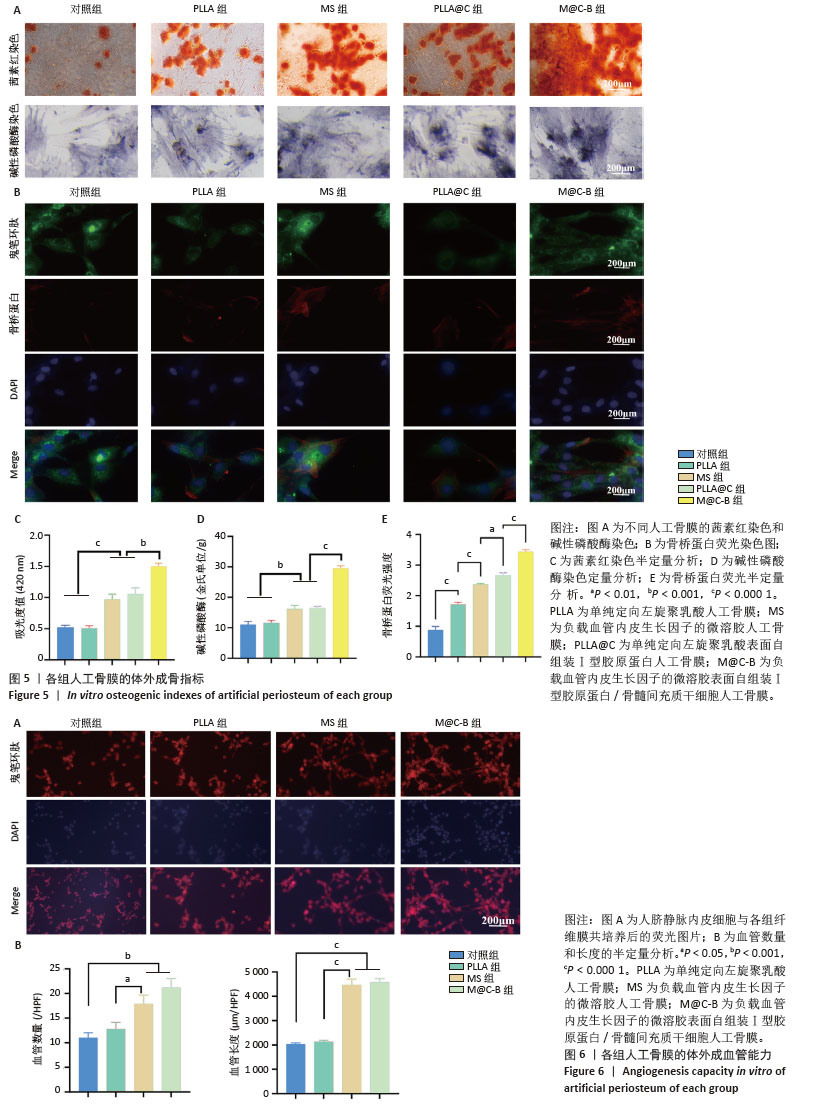
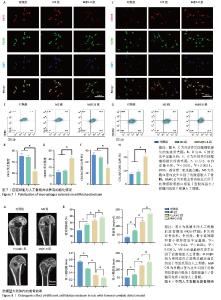
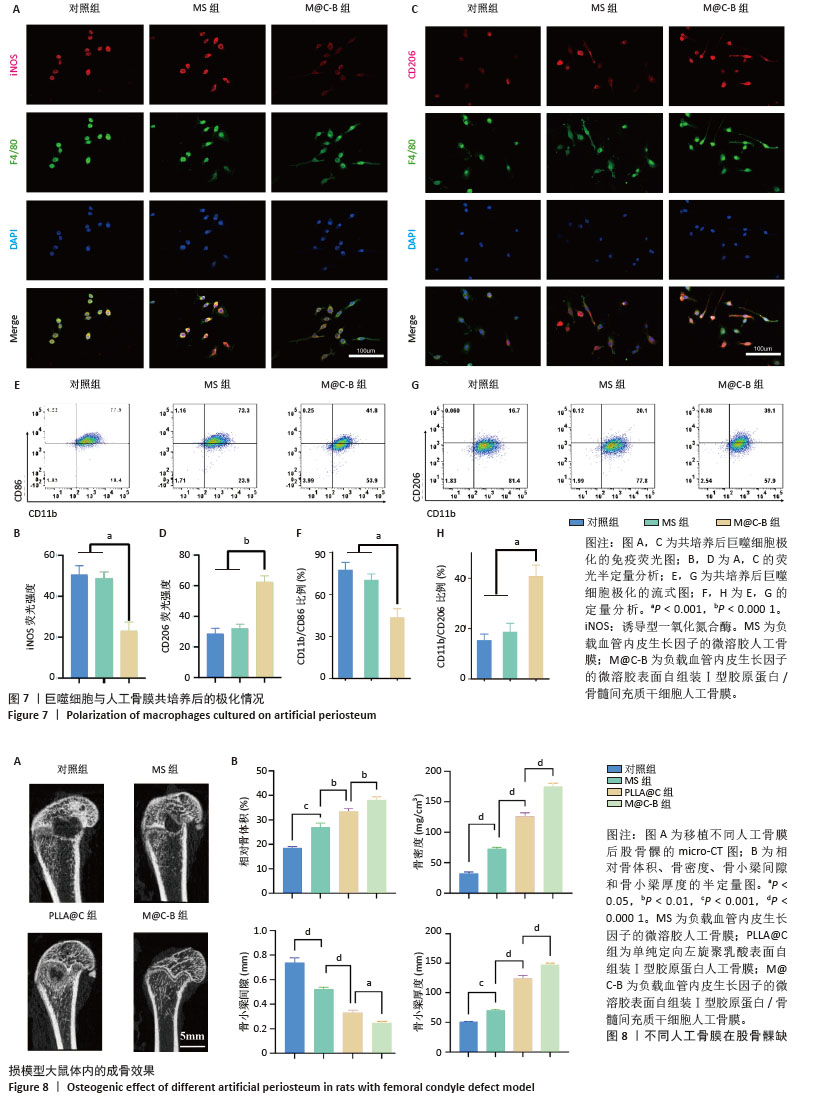
2.7 人工骨膜的体外免疫调节能力 将人工骨膜与骨髓巨噬细胞通过Transwell体系共培养3 d后,采用免疫荧光染色和流式细胞术检测巨噬细胞的不同表型。如图7显示,M@C-B组的M1亚型标记物诱导型一氧化氮合酶的红色荧光强度较弱,明显低于其他组(图7A,B),M2亚型标记物CD206的红色荧光强度较强,明显强于其他组(图7C,D)。采用流式细胞术检测巨噬细胞M1和M2亚型的极化情况(图7E,G),相比于对照组和MS组,M@C-B组CD86和CD11b双阳性(M1亚型)比例为41.8%,比对照组(77.5%)下降一半。M@C-B组CD206和CD11b双阳性(M2亚型)比例明显上升,为39.1%,约为对照组(16.7%)的2.3倍。流式的半定量图与免疫荧光结果类似(图7F,H),证明M@C-B具有一定的免疫协调能力,能一定程度上抑制促炎巨噬细胞的极化,促进巨噬细胞向抗炎表型的转变。 2.8 人工骨膜的体内成骨效果 术后第4周时,使用Micro-CT观察材料的成骨效果,结果如图8A所示,可见人工骨膜(MS组、PLLA@C组、M@C-B组)的植入减小了骨缺损区域的面积,其中MS组虽然具有缓慢释放血管内皮生长因子的作用,然而缺乏骨髓间充质干细胞的免疫调控和种子细胞补充作用,骨缺损区域较Ⅰ型胶原蛋白自组装组(PLLA@C、M@C-B)大,而PLLA@C组中Ⅰ型胶原蛋白在一定程度上能够促进内源性骨髓间充质干细胞的黏附和成骨分化等生物学效应,但伴随体内降解较快,且缺乏炎症调节和血管内皮生长因子持续补充,仍可见损伤局部骨缺损。此外,M@C-B组较其他组协调了免疫炎症调节和血管内皮生长因子促血管化作用,成骨效果最为明显。与对照组相比,植入材料组的骨参数(相对骨体积、骨密度和骨小梁厚度)均明显升高,其中M@C-B组效果最显著。更重要的是,植入人工骨膜后骨小梁间隙减小,说明人工骨膜的应用加速了成骨的过程,减少了骨质疏松(图8B)。以上现象可能是人工骨膜植入增加了细胞的附着面积,更有利于迁移、黏附和分化。 "
| [1] DAI K, DENG S, YU Y, et al. Construction of developmentally inspired periosteum-like tissue for bone regeneration. Bone Res. 2022;10(1):1. [2] ZHANG X, DENG C, QI S. Periosteum Containing Implicit Stem Cells: A Progressive Source of Inspiration for Bone Tissue Regeneration. Int J Mol Sci. 2024;25(4):2162. [3] 张伯宇,王建南.天然高分子材料在人工骨膜领域研究进展[J].现代丝绸科学与技术,2024,39(1):28-32. [4] WU L, GU Y, LIU L, et al. Hierarchical micro/nanofibrous membranes of sustained releasing VEGF for periosteal regeneration. Biomaterials. 2020;227:119555. [5] 杨雨晴,陈志宇.早期短暂M1巨噬细胞在骨组织工程中的作用及应用[J].中国组织工程研究,2024,28(4):594-601. [6] SCHLUNDT C, FISCHER H, BUCHER CH, et al. The multifaceted roles of macrophages in bone regeneration: A story of polarization, activation and time. Acta Biomater. 2021;133:46-57. [7] LU Y, LIAN X, CAO Y, et al. An enhanced tri-layer bionic periosteum with gradient structure loaded by mineralized collagen for guided bone regeneration and in-situ repair. Int J Biol Macromol. 2024; 277(Pt 1):134148. [8] CHEN X, YU B, WANG Z, et al. Progress of Periosteal Osteogenesis: The Prospect of In Vivo Bioreactor. Orthop Surg. 2022;14(9):1930-1939. [9] ZHANG W, WANG N, YANG M, et al. Periosteum and development of the tissue-engineered periosteum for guided bone regeneration. J Orthop Translat. 2022;33:41-54. [10] WANG J, CHEN G, CHEN ZM, et al. Current strategies in biomaterial-based periosteum scaffolds to promote bone regeneration: A review. J Biomater Appl. 2023;37(7):1259-1270. [11] WANG YH, ZHAO CZ, WANG RY, et al. Correction: The crosstalk between macrophages and bone marrow mesenchymal stem cells in bone healing. Stem Cell Res Ther. 2022;13(1):524. [12] 张怡凡,陈悦,周硕,等.HIF-1α通过调控H型血管生成参与牙周炎的发展[J].山西医科大学学报,2024,55(6):746-752. [13] HU Y, HUANG J, CHEN C, et al. Strategies of Macrophages to Maintain Bone Homeostasis and Promote Bone Repair: A Narrative Review. J Funct Biomater. 2022;14(1):18. [14] GOU M, WANG H, XIE H, et al. Macrophages in guided bone regeneration: potential roles and future directions. Front Immunol. 2024;15:1396759. [15] 王寒,邢辉.3D打印生物工程骨支架的相关研究进展[J].中国矫形外科杂志,2024,32(11):1001-1006. [16] WANG J, LIU M, YANG C, et al. Biomaterials for bone defect repair: Types, mechanisms and effects. Int J Artif Organs. 2024;47(2):75-84. [17] SHAW P, DWIVEDI SKD, BHATTACHARYA R, et al. VEGF signaling: Role in angiogenesis and beyond. Biochim Biophys Acta Rev Cancer. 2024; 1879(2):189079. [18] PATEL SA, NILSSON MB, LE X, et al. Molecular Mechanisms and Future Implications of VEGF/VEGFR in Cancer Therapy. Clin Cancer Res. 2023;29(1):30-39. [19] QIN Q, LEE S, PATEL N, et al. Neurovascular coupling in bone regeneration. Exp Mol Med. 2022;54(11):1844-1849. [20] ZHANG J, TONG D, SONG H, et al. Osteoimmunity-Regulating Biomimetically Hierarchical Scaffold for Augmented Bone Regeneration. Adv Mater. 2022;34(36):e2202044. [21] MA S, WANG C, DONG Y, et al. Microsphere-Gel Composite System with Mesenchymal Stem Cell Recruitment, Antibacterial, and Immunomodulatory Properties Promote Bone Regeneration via Sequential Release of LL37 and W9 Peptides. ACS Appl Mater Interfaces. 2022;14(34):38525-38540. [22] 李华德,时强,史晨丽.骨髓干细胞聚乳酸羟基乙酸修复大鼠软骨缺损[J].中国矫形外科杂志,2024,32(13):1222-1228. [23] GAO Y, ZOU Y, SOKOLOWSKEI D, et al. Nr4a1 enhances Wnt4 transcription to promote mesenchymal stem cell osteogenesis and alleviates inflammation-inhibited bone regeneration. Mol Ther. 2024; 32(5):1479-1496. [24] XU Z, WU L, TANG Y, et al. Spatiotemporal Regulation of the Bone Immune Microenvironment via Dam-Like Biphasic Bionic Periosteum for Bone Regeneration. Adv Healthc Mater. 2023;12(1):e2201661. [25] 张洁,田艾.M2巨噬细胞参与骨再生相关信号通路的作用与机制[J].中国组织工程研究,2023,27(2):314-321. [26] LIU Z, ZHU J, LI Z, et al. Biomaterial scaffolds regulate macrophage activity to accelerate bone regeneration. Front Bioeng Biotechnol. 2023;11:1140393. [27] 赵月鑫,陈滨.巨噬细胞极化在骨组织工程免疫研究中的进展[J].中国组织工程研究,2022,26(13):2120-2126. [28] HU K, SHANG Z, YANG X, et al. Macrophage Polarization and the Regulation of Bone Immunity in Bone Homeostasis. J Inflamm Res. 2023;16:3563-3580. [29] WU M, LIU H, ZHU Y, et al. Bioinspired soft-hard combined system with mild photothermal therapeutic activity promotes diabetic bone defect healing via synergetic effects of immune activation and angiogenesis. Theranostics. 2024;14(10):4014-4057. [30] ZHAO Q, LIU X, YU C, et al. Macrophages and Bone Marrow-Derived Mesenchymal Stem Cells Work in Concert to Promote Fracture Healing: A Brief Review. DNA Cell Biol. 2022;41(3):276-284. [31] YOON SJ, KIM SH, CHOI JW, et al. Guided cortical and cancellous bone formation using a minimally invasive technique of BMSC- and BMP-2-laden visible light-cured carboxymethyl chitosan hydrogels. Int J Biol Macromol. 2023;227:641-653. [32] ARTHUR A, GRONTHOS S. Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue. Int J Mol Sci. 2020;21(24):9759. [33] ZHENG ZW, CHEN YH, WU DY, et al. Development of an Accurate and Proactive Immunomodulatory Strategy to Improve Bone Substitute Material-Mediated Osteogenesis and Angiogenesis. Theranostics. 2018;8(19):5482-5500. [34] MAO Y, CHEN Y, LI W, et al. Physiology-Inspired Multilayer Nanofibrous Membranes Modulating Endogenous Stem Cell Recruitment and Osteo-Differentiation for Staged Bone Regeneration. Adv Healthc Mater. 2022; 11(21):e2201457. [35] JIN H, ZHU X, LIU H, et al. Type-I Collagen Polypeptide-Based Composite Nanofiber Membranes for Fast and Efficient Bone Regeneration. ACS Biomater Sci Eng. 2024;10(9):5632-5640. [36] JOVANOVIC M, GUTERMAN-RAM G, MARINI JC. Osteogenesis Imperfecta: Mechanisms and Signaling Pathways Connecting Classical and Rare OI Types. Endocr Rev. 2022;43(1):61-90. [37] CHENG L, CHEN Z, CAI Z, et al. Bioinspired Functional Black Phosphorus Electrospun Fibers Achieving Recruitment and Biomineralization for Staged Bone Regeneration. Small. 2020;16(50):e2005433. [38] HUA X, HOU M, DENG L, et al. Irisin-loaded electrospun core-shell nanofibers as calvarial periosteum accelerate vascularized bone regeneration by activating the mitochondrial SIRT3 pathway. Regen Biomater. 2023;11:rbad096. [39] ZHAO L, LAN W, DONG X, et al. Enhenced cell adhesion on collagen I treated parylene-C microplates. J Biomater Sci Polym Ed. 2021;32(17):2195-2209. [40] LE D, MCMILLAN S. A Novel Distal Biceps Rupture Repair Technique Utilizing a Biocomposite Scaffold. Surg Technol Int. 2023. doi: 10.52198/23.STI.42.OS1656. [41] DONG C, TAN G, ZHANG G, et al. The function of immunomodulation and biomaterials for scaffold in the process of bone defect repair: A review. Front Bioeng Biotechnol. 2023;11:1133995. [42] WANG G, LV Z, WANG T, et al. Surface Functionalization of Hydroxyapatite Scaffolds with MgAlEu-LDH Nanosheets for High-Performance Bone Regeneration. Adv Sci (Weinh). 2022;10(1): e2204234. [43] KIM T, HONG J, KIM J, et al. Two-Dimensional Peptide Assembly via Arene-Perfluoroarene Interactions for Proliferation and Differentiation of Myoblasts. J Am Chem Soc. 2023;145(3): 1793-1802. [44] SUZAWA M, TAMURA Y, FUKUMOTO S, et al. Stimulation of Smad1 transcriptional activity by Ras-extracellular signal-regulated kinase pathway: a possible mechanism for collagen-dependent osteoblastic differentiation. J Bone Miner Res. 2002;17(2):240-248. [45] HUANG J, HAN Q, CAI M, et al. Effect of Angiogenesis in Bone Tissue Engineering. Ann Biomed Eng. 2022;50(8):898-913. [46] 过丽强,赵世天,舒冰.Notch信号通路在骨折愈合过程中作用的研究进展[J].上海交通大学学报(医学版),2023,43(2):222-229. [47] STREET J, BAO M, DEGUZMAN L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99(15):9656-9661. [48] WEIVODA MM, BRADLEY EW. Macrophages and Bone Remodeling. J Bone Miner Res. 2023;38(3):359-369. [49] VERMEULEN S, TAHMASEBI BIRGANI Z, HABIBOVIC P. Biomaterial-induced pathway modulation for bone regeneration. Biomaterials. 2022;283:121431. [50] XUE N, DING X, HUANG R, et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharmaceuticals (Basel). 2022; 15(7):879. [51] 杜锦洲,钱运,范存义.β-磷酸三钙的骨诱导性和组织工程应用研究进展[J].中华骨科杂志,2024,44(13):906-914. [52] ZHANG FF, HAO Y, ZHANG KX, et al. Interplay between mesenchymal stem cells and macrophages: Promoting bone tissue repair. World J Stem Cells. 2024;16(4):375-388. [53] LOCATI M, CURTALE G, MANTOVANI A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev Pathol. 2020;15: 123-147. |
| [1] | Fu Zhenyi, Li Junhao, Zhang Yating, He Yunkai, Liu Junyu, Wei Yunhao, Liu Jiaxin. Schwann cells promote peripheral nerve regeneration: retrospect and prospect [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1236-1246. |
| [2] | Guo Jiachen, Gao Jun, Dai Wenhao, Liao Huayuan, Jiang You, Zhang Xi . Effect of compressive stress microenvironment on cytokines during fracture healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 908-916. |
| [3] | Cao Wenqi, Feng Xiuzhi, Zhao Yi, Wang Zhimin, Chen Yiran, Yang Xiao, Ren Yanling. Effect of macrophage polarization on osteogenesis-angiogenesis coupling in type 2 diabetic osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 917-925. |
| [4] | Wang Ying, Wang Yawen, Xu Yingjie, Wang Yuanfei, Wu Tong. Preparation of polycaprolactone/low molecular weight fucoidan nanofibers by emulsion electrospinning and assessment of their biocompatibility [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 433-442. |
| [5] | Zhou Shibo, Yu Xing, Chen Hailong, Xiong Yang. Nanocrystalline collagen-based bone combined with Bushen Zhuangjin Decoction repairs bone defects in osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 354-361. |
| [6] | Xie Peisen, Guan Zhenpeng, Wei Xianjie, Zhang Keshi, Kang Qingyuan, Xiao Wentao, Guo Xiaoshuai. Xie Peisen, Guan Zhenpeng, Wei Xianjie, Zhang Keshi, Kang Qingyuan, Xiao Wentao, Guo Xiaoshuai [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 375-383. |
| [7] | Yuan Qian, Zhang Hao, Pang Jie. Characterization and biological properties of naringin-loaded chitosan/beta-tricalcium phosphate scaffold [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 424-432. |
| [8] | Xu Haichao, Luo Lihua, Pan Yihuai. Application and progress of dental pulp stem cells and their derivatives in dental pulp regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 153-162. |
| [9] | Zhang Zhaowei, Chen Ouzile, Bai Mingru, Wang Chenglin. Therapeutic potential of bioactive substances secreted by dental mesenchymal stem cells for bone repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 163-174. |
| [10] | Sun Zhanpeng, Liu Sen, Shi Ling, Chen Kaiyuan, Song Meichen, Wu Yan, Yu Jing. Bone marrow mesenchymal stem cell nanovesicles fusion neutrophil apoptotic bodies promote skin wound healing in diabetic mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 34-42. |
| [11] | Chen Qiheng, Weng Tujun, Peng Jiang. Effect of dimethylglyoxal glycine on osteogenic, adipogenesis differentiation, and mitophagy of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 50-57. |
| [12] | Ma Wenjing, Zhang Jinyu, Jiang Mingxia, Xiu Bingshui, Bai Rui, Liu Yuhan, Chen Xuyi, Yuan Zengqiang, Liu Zhiqiang. Scaffold-free three-dimensional human umbilical cord mesenchymal stem cell secretome repairs mouse skin injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 68-77. |
| [13] | Zhang Tingting, Li Yalong, Yue Haodi, Li Yanjun, Geng Xiwen, Zhang Yuwei, Liu Xiaozhuan. Protection of exosomes derived from bone marrow mesenchymal stem cells of different mouse ages on radiation-induced lung injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 1-9. |
| [14] | Yao Lijie, Yan Yuying, Chen Siyu, Wang Yuanfei, Wu Tong. Nanofibers with gradient deposition of endothelial cell derived matrix particles modulate the behavior of Schwann cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-10. |
| [15] | Wang Wentao, Hou Zhenyang, Wang Yijun, Xu Yaozeng. Apelin-13 alleviates systemic inflammatory bone loss by inhibiting macrophage M1 polarization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1548-1555. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||