Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (1): 10-20.doi: 10.12307/2026.539
Previous Articles Next Articles
Senescence of human bone marrow mesenchymal stromal cells with increasing age is not dependent on the mediation of endogenous retroviruses
Wang Yaping1, Gao Tianyun2, Wang Bin1, 2
- 1Clinical Stem Cell Center, Nanjing Drum Tower Hospital, Nanjing Drum Tower Hospital Clinical College of Nanjing University of Chinese Medicine, Nanjing 210008, Jiangsu Province, China; 2Clinical Stem Cell Center, Nanjing Drum Tower Hospital, Affiliated Hospital of Nanjing University Medical School, Nanjing 210008, Jiangsu Province, China
-
Received:2024-12-07Accepted:2025-01-25Online:2026-01-08Published:2025-06-12 -
Contact:Wang Bin, MD, Professor, Researcher, Clinical Stem Cell Center, Nanjing Drum Tower Hospital, Nanjing Drum Tower Hospital Clinical College of Nanjing University of Chinese Medicine, Nanjing 210008, Jiangsu Province, China; Clinical Stem Cell Center, Nanjing Drum Tower Hospital, Affiliated Hospital of Nanjing University Medical School, Nanjing 210008, Jiangsu Province, China -
About author:Wang Yaping, Master candidate, Clinical Stem Cell Center, Nanjing Drum Tower Hospital, Nanjing Drum Tower Hospital Clinical College of Nanjing University of Chinese Medicine, Nanjing 210008, Jiangsu Province, China -
Supported by:National Natural Science Foundation of China (General Program), No. 82070459 and No. 82270701 (to WB)
CLC Number:
Cite this article
Wang Yaping, Gao Tianyun, Wang Bin. Senescence of human bone marrow mesenchymal stromal cells with increasing age is not dependent on the mediation of endogenous retroviruses[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 10-20.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

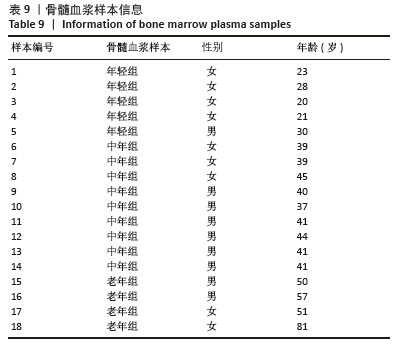
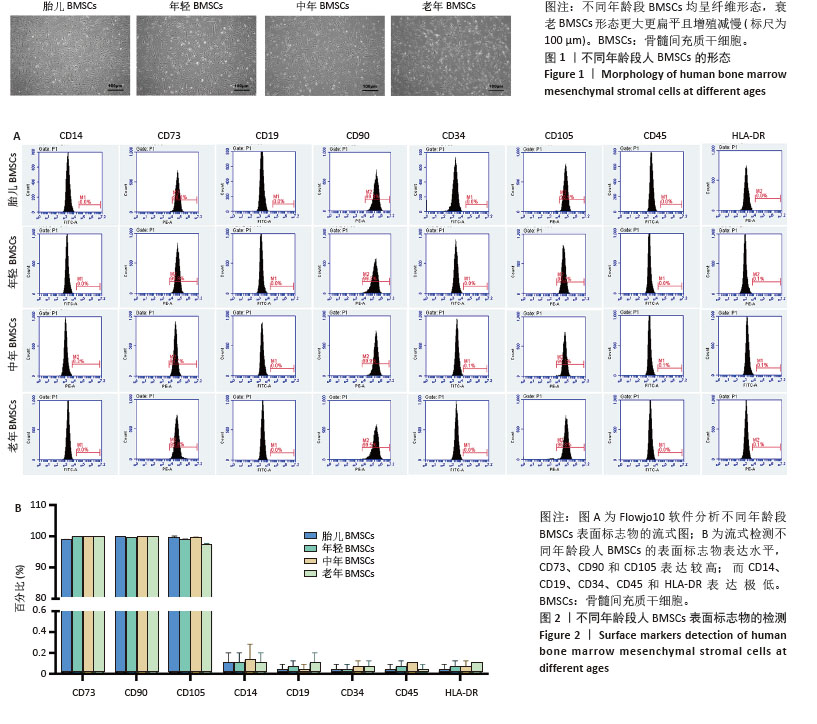
2.1 不同年龄段BMSCs的形态 细胞接种在6孔板中,用完全培养基培养24 h后细胞贴壁生长,细胞呈梭形、平行或漩涡状排列。随着年龄的增长,细胞形态发生改变,衰老BMSCs的大小和粒度显著增加,细胞变得更加扁平,见图1。 2.2 不同年龄段BMSCs的表面标志物 不同年龄段BMSCs表面标志物CD73、CD90和CD105呈阳性(> 95%),CD14、CD19、CD34、CD45和HLA-DR呈阴性(< 2%),符合国际干细胞研究学会和国际细胞治疗协会定义的间充质干细胞的通用标准[52-55],且不同年龄段BMSCs具有相似的表面标志物,表明BMSCs增龄衰老不会影响其典型表面标志物的表达,见图2。 "
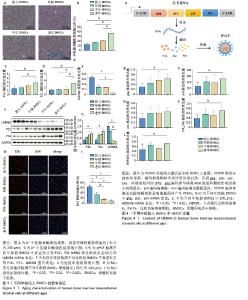
2.3 老年供者来源BMSCs呈现显著的衰老表型 β-半乳糖苷酶染色结果显示,不同年龄段BMSCs的β-半乳糖苷酶阳性率不同,随着供者年龄的增长,衰老BMSCs中β-半乳糖苷酶阳性率更高,表现出细胞衰老程度增加。此外,用qPCR检测不同年龄段BMSCs中典型衰老标志物P16、P21[56]、LMNB1 mRNA表达,结果显示,老年BMSCs中P16、P21 mRNA表达升高,核形态标志物LMNB1 mRNA表达下降,与衰老细胞的表型一致。为了进一步准确表征不同年龄段BMSCs的衰老程度,用免疫印迹法检测了不同年龄段BMSCs中P16、P21、LMNB1蛋白表达,结果显示,老年BMSCs中P16、P21蛋白表达升高,LMNB1蛋白表达下降,与qPCR结果相一致。细胞增殖也是细胞衰老的一个重要指标,细胞以分裂的形式进行增殖,在细胞增殖过程中会发生DNA复制,EdU与DNA结合,并传到复制的DNA中,可以通过检测DNA的合成情况,来判定细胞增殖情况[57]。随着BMSCs的衰老,EdU标记阳性细胞率显著降低,表明细胞增殖能力减弱。 以上结果表明,不同年龄段供者来源BMSCs的衰老表型有显著差异,来源于老年供者的BMSCs表现出更明显的衰老表型,见图3。 2.4 不同年龄段BMSCs中均存在HERVK HERVK是由编码基因gag、pro、pol、env和两端的重复序列LTR构成,能够被转录、翻译、包装为RVLPs。为研究BMSCs的衰老是否与HERVK有关,使用qPCR检测不同年龄段BMSCs中HERVK含量,见图4。结果显示,不同年龄段BMSCs中均检测到HERVK,且HERVK编码基因gag、pol、env和末端重复序列LTR基因均无显著性差异。HERVK6是一种内源性反转录病毒包膜蛋白,在感染早期介导受体识别和膜融合,用以检测RVLPs的形成。HERVK6的qPCR结果显示,不同年龄段BMSCs中的RVLPs也没有显著差异。表明在不同年龄段BMSCs中存在HERVK,随着细胞衰老,BMSCs中的HERVK表达并未增加。"
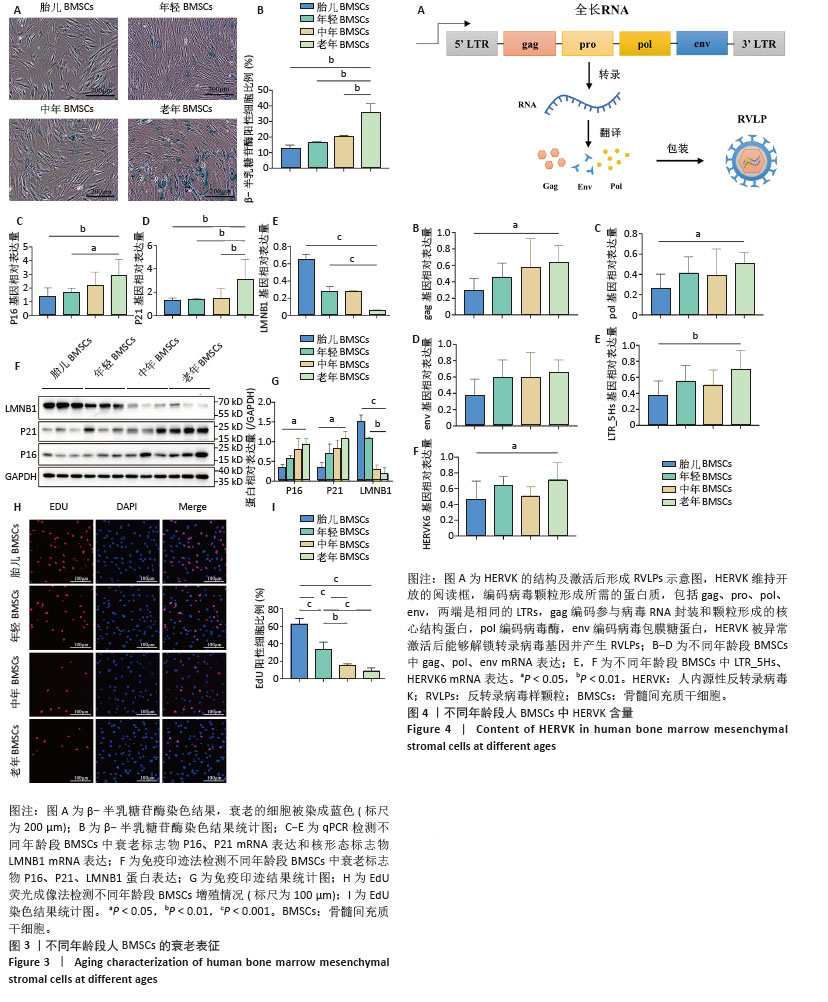

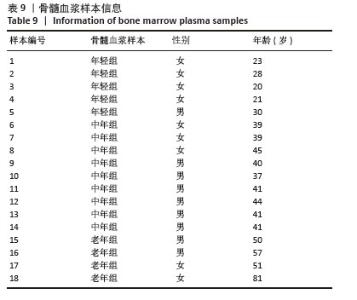
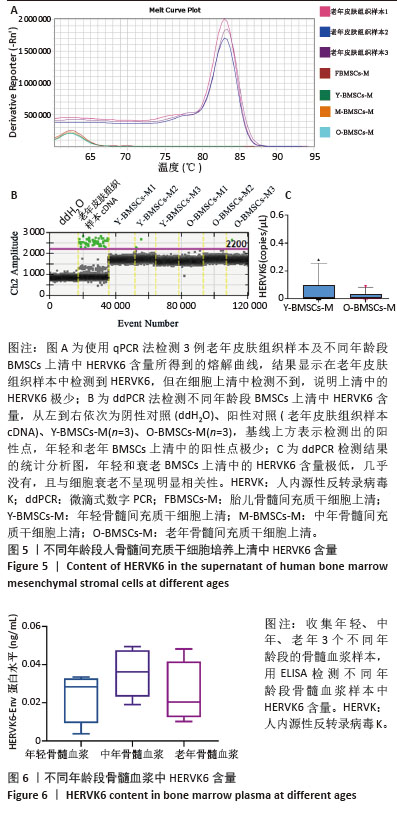
2.5 衰老BMSCs中HERVK未被激活 为了进一步研究BMSCs衰老表型是否由HERVK激活引起,首先通过qPCR法检测细胞培养上清样本HERVK6表达,以老年皮肤组织样本作为阳性对照,结果显示在老年皮肤组织样本中检测到HERVK6,然而在细胞培养上清中未检测到此病毒颗粒,这可能是上清中的病毒颗粒含量低于普通qPCR仪的检测限,也可能是BMSCs中的HERVK未被激活释放。为了能更准确地研究上清样本中的病毒含量,使用了灵敏度更高的液滴式数字PCR (ddPCR),相较于qPCR能实现绝对定量、精准定量和高度重复,且不易受扩增效率的影响。ddPCR结果显示不同年龄段供者来源BMSCs培养上清中HERVK6含量极低,几乎没有,并且随着BMSCs的衰老,上清中HERVK6含量未明显升高,不与衰老呈相关性,见图5。结果表明,在年轻和老年BMSCs中HERVK均未被异常激活。ddPCR系统检测到的微量拷贝数的病毒颗粒可能是由于细胞破裂或者细胞凋亡后释放到上清中,而并不是HERVK被激活的信号。 2.6 不同年龄段骨髓微环境中HERVK6的检测 为了进一步验证ddPCR结果的可信度,对不同年龄段骨髓微环境中的HERVK6含量进行检测,收集了不同年龄段人骨髓血浆样本,通过Elisa试剂盒检测不同年龄段人骨髓血浆样本中HERVK6的表达,血浆样本信息如表9所示。不同年龄段骨髓血浆样本中HERVK6未明显增加,见图6。在不同年龄段骨髓微环境中HERVK的表达与年龄没有呈现出显著的相关性,进一步验证了随着BMSCs的衰老,HERVK未被异常激活释放RVLPs到细胞外,表明在正常生理衰老条件下,BMSCs的衰老并非由HERVK的激活引起。"
| [1] BLACKBURN EH, EPEL ES, LIN J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science (New York, NY). 2015;350(6265):1193-1198. [2] BARTEK J, FALCK J, LUKAS J. CHK2 kinase--a busy messenger. Nat Rev Mol Cell Biol. 2001;2(12):877-886. [3] HERNANDEZ-SEGURA A, NEHME J, DEMARIA M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018;28(6):436-453. [4] VASILEIOU PVS, EVANGELOU K, VLASIS K, et al. Mitochondrial Homeostasis and Cellular Senescence. Cells. 2019;8(7):686. [5] LÓPEZ-OTÍN C, BLASCO MA, PARTRIDGE L, et al. The hallmarks of aging. Cell. 2013;153(6):1194-1217. [6] LIU X, LIU Z, WU Z, et al. Resurrection of endogenous retroviruses during aging reinforces senescence. Cell. 2023;186(2):287-304.e26. [7] YANG JH, HAYANO M, GRIFFIN PT, et al. Loss of epigenetic information as a cause of mammalian aging. Cell. 2023;186(2):305-326.e27. [8] LÓPEZ-OTÍN C, BLASCO MA, PARTRIDGE L, et al. Hallmarks of aging: An expanding universe. Cell. 2023;186(2):243-278. [9] ÁLVAREZ-SATTA M, BERNA-ERRO A, CARRASCO-GARCIA E, et al. Relevance of oxidative stress and inflammation in frailty based on human studies and mouse models. Aging (Albany NY). 2020;12(10):9982-9999. [10] MOUSTOGIANNIS A, PHILIPPOU A, TASO O, et al. The Effects of Muscle Cell Aging on Myogenesis. Int J Mol Sci. 2021;22(7):3721. [11] LI CJ, XIAO Y, SUN YC, et al. Senescent immune cells release grancalcin to promote skeletal aging. Cell Metab. 2021;33(10):1957-1973.e6. [12] PALMER AK, GUSTAFSON B, KIRKLAND JL, et al. Cellular senescence: at the nexus between ageing and diabetes. Diabetologia. 2019;62(10): 1835-1841. [13] ACHA B, CORROZA J, SÁNCHEZ-RUIZ DE GORDOA J, et al. Association of Blood-Based DNA Methylation Markers With Late-Onset Alzheimer Disease: A Potential Diagnostic Approach. Neurology. 2023;101(23):e2434-e2447. [14] KIP E, PARR-BROWNLIE LC. Reducing neuroinflammation via therapeutic compounds and lifestyle to prevent or delay progression of Parkinson’s disease. Ageing Res Rev. 2022;78:101618. [15] KRITSILIS M, V RIZOU S, KOUTSOUDAKI PN, et al. Ageing, Cellular Senescence and Neurodegenerative Disease. Int J Mol Sci. 2018;19(10):2937. [16] CHEN MS, LEE RT, GARBERN JC. Senescence mechanisms and targets in the heart. Cardiovasc Res. 2022;118(5):1173-1187. [17] PILLERON S, SOTO-PEREZ-DE-CELIS E, VIGNAT J, et al. Estimated global cancer incidence in the oldest adults in 2018 and projections to 2050. Int J Cancer. 2021;148(3):601-608. [18] GUO J, HUANG X, DOU L, et al. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct Target Ther. 2022;7(1):391. [19] REVUELTA M, MATHEU A. Autophagy in stem cell aging. Aging Cell. 2017; 16(5):912-915. [20] REN R, OCAMPO A, LIU GH, et al. Regulation of Stem Cell Aging by Metabolism and Epigenetics. Cell Metab. 2017;26(3):460-474. [21] DENG P, YUAN Q, CHENG Y, et al. Loss of KDM4B exacerbates bone-fat imbalance and mesenchymal stromal cell exhaustion in skeletal aging. Cell Stem Cell. 2021;28(6):1057-1073.e7. [22] LI X, LUO X, HE Y, et al. Micronano Titanium Accelerates Mesenchymal Stem Cells Aging through the Activation of Senescence-Associated Secretory Phenotype. ACS Nano. 2023;17(22):22885-22900. [23] GOODELL MA, RANDO TA. Stem cells and healthy aging. Science. 2015; 350(6265):1199-1204. [24] WANG Y, GAO T, WANG B. Application of mesenchymal stem cells for anti-senescence and clinical challenges. Stem Cell Res Ther. 2023;14(1):260. [25] LI H, DAI H, LI J. Immunomodulatory properties of mesenchymal stromal/stem cells: The link with metabolism. J Adv Res. 2023;45:15-29. [26] 张宇宁.HK1通过与TERT结合抑制端粒酶活性调控肺腺癌细胞细胞衰老[D].长春:吉林大学,2023. [27] VOLKOVA MV, SHEN N, POLYANSKAYA A, et al. Tissue-Oxygen-Adaptation of Bone Marrow-Derived Mesenchymal Stromal Cells Enhances Their Immunomodulatory and Pro-Angiogenic Capacity, Resulting in Accelerated Healing of Chemical Burns. Int J Mol Sci. 2023;24(4):4102. [28] WANG Y, LI W, GUO Y, et al. Mitochondria Transplantation to Bone Marrow Stromal Cells Promotes Angiogenesis During Bone Repair. Adv Sci (Weinh). 2024;11(39):e2403201. [29] POUREBRAHIM R, HEINZ MONTOYA R, ALANIZ Z, et al. Mdm2/p53 levels in bone marrow mesenchymal stromal cells are essential for maintaining the hematopoietic niche in response to DNA damage. Cell Death Dis. 2023; 14(6):371. [30] WEI FL, ZHAI Y, WANG TF, et al. Stem cell-homing biomimetic hydrogel promotes the repair of osteoporotic bone defects through osteogenic and angiogenic coupling. Sci Adv. 2024;10(44):eadq6700. [31] BAI B, LIU Y, HUANG J, et al. Tolerant and Rapid Endochondral Bone Regeneration Using Framework-Enhanced 3D Biomineralized Matrix Hydrogels. Adv Sci (Weinh). 2024;11(9):e2305580. [32] LI DX, MA Z, SZOJKA AR, et al. Non-hypertrophic chondrogenesis of mesenchymal stem cells through mechano-hypoxia programing. J Tissue Eng. 2023;14:20417314231172574. [33] PACKHAM DK, FRASER IR, KERR PG, et al. Allogeneic Mesenchymal Precursor Cells (MPC) in Diabetic Nephropathy: A Randomized, Placebo-controlled, Dose Escalation Study. EBioMedicine. 2016;12:263-269. [34] VENKATARAMANA NK, KUMAR SK, BALARAJU S, et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl Res. 2010;155(2):62-70. [35] GRAJEK S, POPIEL M, GIL L, et al. Influence of bone marrow stem cells on left ventricle perfusion and ejection fraction in patients with acute myocardial infarction of anterior wall: randomized clinical trial: Impact of bone marrow stem cell intracoronary infusion on improvement of microcirculation. Eur Heart J. 2010;31(6):691-702. [36] SYKOVÁ E, RYCHMACH P, DRAHORÁDOVÁ I, et al. Transplantation of Mesenchymal Stromal Cells in Patients With Amyotrophic Lateral Sclerosis: Results of Phase I/IIa Clinical Trial. Cell Transplant. 2017;26(4):647-658. [37] SHEN J, TSAI YT, DIMARCO NM, et al. Transplantation of mesenchymal stem cells from young donors delays aging in mice. Sci Rep. 2011;1:67. [38] FREIJE JM, LÓPEZ-OTÍN C. Reprogramming aging and progeria. Curr Opin Cell Biol. 2012;24(6):757-764. [39] MANI C, REDDY PH, PALLE K. DNA repair fidelity in stem cell maintenance, health, and disease. Biochim Biophys Acta Mol Basis Dis. 2020;1866(4):165444. [40] BAI L, LIU Y, ZHANG X, et al. Osteoporosis remission via an anti-inflammaging effect by icariin activated autophagy. Biomaterials. 2023;297:122125. [41] SU X, ZHANG H, LEI F, et al. Epigenetic therapy attenuates oxidative stress in BMSCs during ageing. J Cell Mol Med. 2022;26(2):375-384. [42] MATTEUCCI C, BALESTRIERI E, ARGAW-DENBOBA A, et al. Human endogenous retroviruses role in cancer cell stemness. Semin Cancer Biol. 2018;53:17-30. [43] KONG X, LI R, CHEN M, et al. Endogenous retrovirus HERVH-derived lncRNA UCA1 controls human trophoblast development. Proc Natl Acad Sci U S A. 2024;121(12):e2318176121. [44] KREBS AS, LIU HF, ZHOU Y, et al. Molecular architecture and conservation of an immature human endogenous retrovirus. Nat Commun. 2023;14(1):5149. [45] DOPKINS N, NIXON DF. Activation of human endogenous retroviruses and its physiological consequences. Nat Rev Mol Cell Biol. 2024;25(3):212-222. [46] HU M, XING L, ZHANG L, et al. NAP1L2 drives mesenchymal stem cell senescence and suppresses osteogenic differentiation. Aging Cell. 2022; 21(2):e13551. [47] 袁雅红,赵珊珊,王小莉,等.HIV P55-gag蛋白促进骨髓间充质干细胞的老化[J].生物医学工程研究,2017,36(1):52-56+61. [48] 王龙,蒋航,陈图南,等.TAS102选择性抑制ATRX缺失胶质瘤细胞的实验研究[J].第三军医大学学报,2019,41(24):2393-2400. [49] XIAO X, XU M, YU H, et al. Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal Transduct Target Ther. 2021;6(1):354. [50] 张晓雯,徐康彦,王帮华,等.蝙蝠葛碱对恶性胶质瘤U87细胞的抑制及分子机制研究[J].湖北科技学院学报(医学版),2022,36(6):476-480. [51] 吴美燕.FOXK1与FHL2在大肠癌的增殖、EMT、侵袭和转移中的相互作用[D].广州:南方医科大学,2017. [52] IVANOVSKI S, HAN P, PETERS OA, et al. The Therapeutic Use of Dental Mesenchymal Stem Cells in Human Clinical Trials. J Dent Res. 2024;103(12): 1173-1184. [53] 冯乙芮,高天芸,王亚萍,等.白细胞介素10工程化修饰人脐带间充质干细胞优效治疗炎症性肠病[J].中国组织工程研究,2025,29(23): 4878-4887. [54] 王慧慧,吴情,赵玉梅.基于RNA测序的SHED体外连续扩增后差异表达基因的研究[J].口腔颌面外科杂志,2023,33(4):211-218. [55] 王留娣,刘威,谢园园,等.三种人胎盘组织来源间充质干细胞的生物学特性[J].中国组织工程研究,2019,23(9):1377-1383. [56] STOLZING A, JONES E, MCGONAGLE D, et al. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129(3):163-173. [57] 纪佳男.抑制抗氧化通路与ATR抑制剂在癌细胞中的协同致死作用及其机理研究[D].长春:吉林大学,2022. [58] LIU F, YUAN Y, BAI L, et al. LRRc17 controls BMSC senescence via mitophagy and inhibits the therapeutic effect of BMSCs on ovariectomy-induced bone loss. Redox Biol. 2021;43:101963. [59] YANG Q, ZOU Y, WEI X, et al. PTP1B knockdown alleviates BMSCs senescence via activating AMPK-mediated mitophagy and promotes osteogenesis in senile osteoporosis. Biochim Biophys Acta Mol Basis Dis. 2023;1869(7):166795. [60] LI G, ZHU Q, WANG B, et al. Rejuvenation of Senescent Bone Marrow Mesenchymal Stromal Cells by Pulsed Triboelectric Stimulation. Adv Sci (Weinh). 2021;8(18):e2100964. [61] ZHOU S, GREENBERGER JS, EPPERLY MW, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7(3):335-343. [62] MADSEN SD, RUSSELL KC, TUCKER HA, et al. Decoy TRAIL receptor CD264: a cell surface marker of cellular aging for human bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther. 2017;8(1):201. [63] 武泽明,刘晓倩,刘光慧.内源性逆转录病毒复活作为衰老的新型生物标志物和驱动力[J].科学通报,2024,69(Z2):4163-4165. [64] GARCIA-MONTOJO M, FATHI S, NORATO G, et al. Inhibition of HERV-K (HML-2) in amyotrophic lateral sclerosis patients on antiretroviral therapy. J Neurol Sci. 2021;423:117358. [65] DEMBNY P, NEWMAN AG, SINGH M, et al. Human endogenous retrovirus HERV-K(HML-2) RNA causes neurodegeneration through Toll-like receptors. JCI Insight. 2020;5(7):e131093. [66] EHLHARDT S, SEIFERT M, SCHNEIDER J, et al. Human endogenous retrovirus HERV-K(HML-2) Rec expression and transcriptional activities in normal and rheumatoid arthritis synovia. J Rheumatol. 2006;33(1):16-23. [67] 张保虎,刘晓倩,武泽明,等.内源性逆转录病毒在人类健康及衰老中的作用和机制研究[J].中国基础科学,2024,26(1):14-21. [68] 曾晨叶,花瑞,王钊.衰老细胞清除策略与CAR细胞疗法在抗衰老治疗中的应用[J].中国生物化学与分子生物学报,2024,40(9):1197-1204. [69] LI C, WEI GJ, XU L, et al. The involvement of senescence induced by the telomere shortness in the decline of osteogenic differentiation in BMSCs. Eur Rev Med Pharmacol Sci. 2017;21(5):1117-1124. [70] BANIMOHAMAD-SHOTORBANI B, KAHROBA H, SADEGHZADEH H, et al. DNA damage repair response in mesenchymal stromal cells: From cellular senescence and aging to apoptosis and differentiation ability. Ageing Res Rev. 2020;62:101125. [71] XU Y, CHANG L, CHEN Y, et al. USP26 Combats Age-Related Declines in Self-Renewal and Multipotent Differentiation of BMSC by Maintaining Mitochondrial Homeostasis. Adv Sci (Weinh). 2024;11(44):e2406428. [72] ZHANG H, XU R, LI B, et al. LncRNA NEAT1 controls the lineage fates of BMSCs during skeletal aging by impairing mitochondrial function and pluripotency maintenance. Cell Death Differ. 2022;29(2):351-365. [73] ZHAO Y, HE J, QIU T, et al. Epigenetic therapy targeting bone marrow mesenchymal stem cells for age-related bone diseases. Stem Cell Res Ther. 2022;13(1):201. [74] BRUNET A, GOODELL MA, RANDO TA. Ageing and rejuvenation of tissue stem cells and their niches. Nat Rev Mol Cell Biol. 2023;24(1):45-62. [75] DENG J, OUYANG P, LI W, et al. Curcumin Alleviates the Senescence of Canine Bone Marrow Mesenchymal Stem Cells during In Vitro Expansion by Activating the Autophagy Pathway. Int J Mol Sci. 2021;22(21):11356. [76] ZHANG D, LIN J, WANG Y, et al. Effects of resveratrol on aging of mesenchymal stem cells and its mechanism. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019;48(6):617-624. [77] 汪子铃.人参皂苷Rg1调控Keap1-NRF2-ARE通路延缓骨髓间充质干细胞衰老的机制研究[D].重庆:重庆医科大学,2021. [78] KANG X, CHEN L, YANG S, et al. Zuogui Wan slowed senescence of bone marrow mesenchymal stem cells by suppressing Wnt/β-catenin signaling. J Ethnopharmacol. 2022;294:115323. [79] 施伟丽,刘珊珊,常红波,等.血管内皮生长因子联合碱性成纤维细胞生长因子改善骨髓间充质干细胞复制性衰老[J].中国组织工程研究, 2024,28(31):4958-4963. [80] 张天喆.人多能性干细胞中内源性逆转录病毒元件调控宿主基因表达的研究[D].武汉:武汉大学,2023. |
| [1] | Zhang Haiwen, Zhang Xian, Xu Taichuan, Li Chao. Bibliometric and visual analysis of the research status and trends of senescence in osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1580-1591. |
| [2] | Lai Jiaming, , Song Yuling, Chen Zixi, Wei Jinghuan, Cai Hao, , Li Guoquan, . Screening of diagnostic markers for endothelial cell Senescence in mice with radiation-induced heart disease and analysis of immune infiltration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1450-1463. |
| [3] | Jia Ruiying, Mei Jie, He Qiang, Li Dan, Sun Xin, Qian Weiqing, Liu Zhen. Catalpol from Rehmannia glutinosa regulates senescence in ATDC5 chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(34): 5467-5472. |
| [4] | Yuan Changshen, Liao Shuning, Li Zhe, Wu Siping, Chen Lewei, Liu Jinyi, Li Yanhong, Duan Kan. Machine learning combined with bioinformatics to identify and validate key genes for cellular senescence in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(20): 3196-3202. |
| [5] | Xie Ting, Liu Tingting, Zeng Xuehui, Li Yamin, Zhou Panghu, Yi Nianhua. Vitamin D3 attenuates high-glucose exposure-induced oxidative stress to promote osteogenic differentiation of human umbilical cord mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(19): 2981-2987. |
| [6] | Zhan Yuanbo, Liu Xinpeng, Xu Wenxia, Miao Nan, Mu Sen, Zhang Ruimin, Li Ying. Expression of histone deacetylase 9 in bone marrow mesenchymal stem cells during senescence [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(30): 4762-4766. |
| [7] | Chen Sheng, Yang Yan, Ding Liang, Ye Chuanjin, Zhang Lei, Xia Shu, Li Huiling, Huang Xiaofeng. Establishment and identification of an immortalized oral cancer-related fibroblast line [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3808-3813. |
| [8] | He Fan, Lai Shuaiwei, Zhang Shasha, Dong Fan, Amber Naz, Haniya Mazhar, He Lin, Zhu Hongxin. Autophagy-related gene Rubicon deficiency inhibits cellular senescence in the heart [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3897-3902. |
| [9] | Mai Liping, He Guodong, Chen Shaoxian, Zhu Jiening, Hou Xinghua, Zhang Mengzhen, Li Xiaohong. Expression of aldehyde dehydrogenase 3B1 during human bone marrow mesenchymal stem cells senescence [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 40-44. |
| [10] | Lai Shuaiwei, Zhang Shasha, Liu Xiaoyun, Haniya Mazhar, Amber Naz, He Lin, Zhu Hongxin. Ultraviolet resistance-associated gene (Uvrag) deficiency promotes cellular senescence in the heart [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(14): 2241-2246. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||