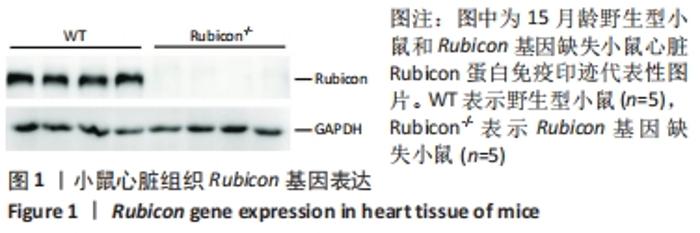
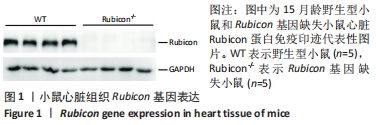
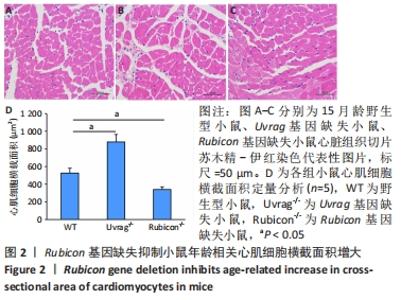
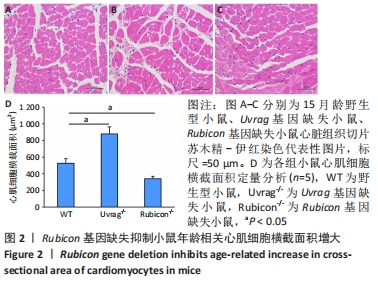
Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (24): 3897-3902.doi: 10.12307/2022.573
Previous Articles Next Articles
Autophagy-related gene Rubicon deficiency inhibits cellular senescence in the heart
He Fan, Lai Shuaiwei, Zhang Shasha, Dong Fan, Amber Naz, Haniya Mazhar, He Lin, Zhu Hongxin
- Bio-X Institutes, Key Laboratory for the Genetics of Developmental and Neuropsychiatric Disorders, Ministry of Education, Shanghai Jiao Tong University, Shanghai 200240, China
-
Received:2021-04-07Accepted:2021-05-21Online:2022-08-28Published:2022-01-24 -
Contact:Zhu Hongxin, Associate researcher, Bio-X Institutes, Key Laboratory for the Genetics of Developmental and Neuropsychiatric Disorders, Ministry of Education, Shanghai Jiao Tong University, Shanghai 200240, China -
About author:He Fan, Master, Bio-X Institutes, Key Laboratory for the Genetics of Developmental and Neuropsychiatric Disorders, Ministry of Education, Shanghai Jiao Tong University, Shanghai 200240, China -
Supported by:the National Natural Science Foundation of China, No. 81974024 (to ZHX); the Natural Science Foundation of Shanghai, No. 16ZR1418200 (to ZHX)
CLC Number:
Cite this article
He Fan, Lai Shuaiwei, Zhang Shasha, Dong Fan, Amber Naz, Haniya Mazhar, He Lin, Zhu Hongxin. Autophagy-related gene Rubicon deficiency inhibits cellular senescence in the heart[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3897-3902.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
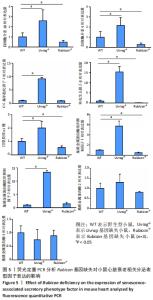
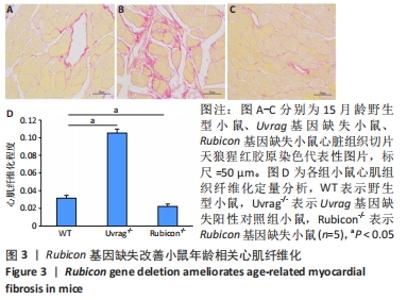
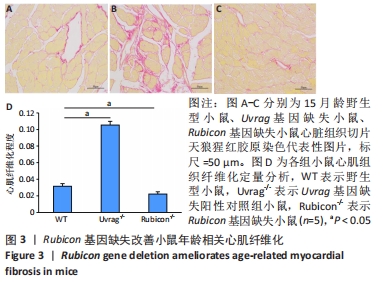
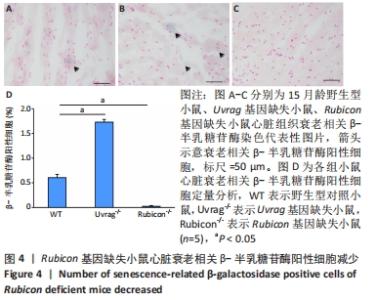
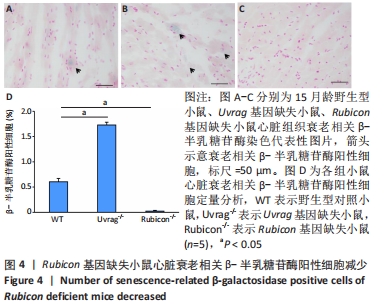
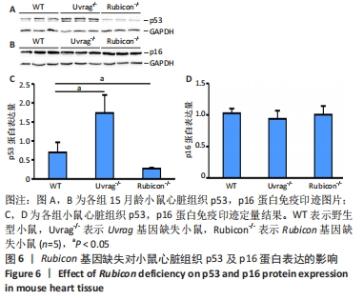
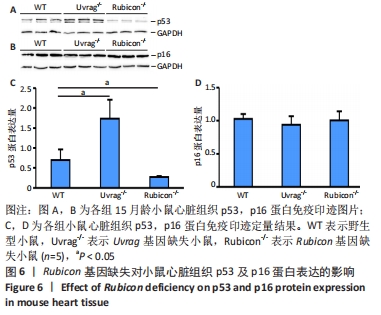
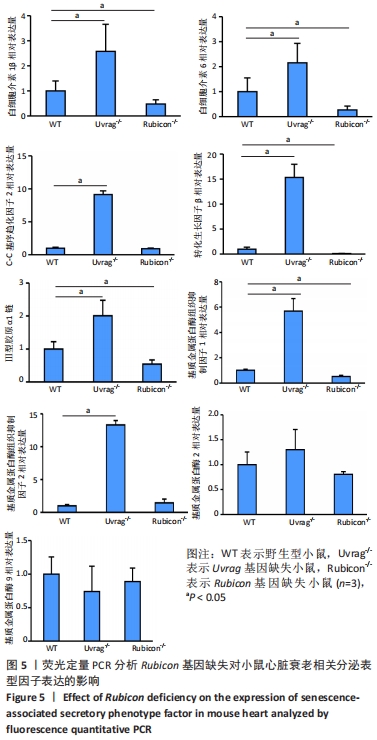
2.4 Rubicon基因缺失降低心脏SASP因子表达 分泌SASP因子是衰老细胞的一个重要特征,也常用作细胞衰老的标志物[33]。利用荧光定量PCR方法检测Rubicon基因缺失对心脏组织SASP因子表达的影响,结果显示15月龄Rubicon基因缺失小鼠心脏白细胞介素1β、白细胞介素6、转化生长因子β和Ⅲ型胶原α1链mRNA表达较野生型对照组显著下调;C-C基序趋化因子2 mRNA表达与野生型对照组无显著差异,此外,15月龄Rubicon基因缺失小鼠组织基质金属蛋白酶组织抑制因子1 mRNA表达量较相应野生型对照组显著下调,而基质金属蛋白酶组织抑制因子2、基质金属蛋白酶2和基质金属蛋白酶9的mRNA表达量无显著变化。以上结果表明Rubicon基因缺失抑制心脏SASP因子表达,见图5。"
| [1] FLORA GD, NAYAK MK. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. Curr Pharm Des. 2019;25(38):4063-4084. [2] SHAKERI H, LEMMENS K, GEVAERT AB, et al. Cellular Senescence Links Aging and Diabetes in Cardiovascular Disease. Am J Physiol Heart Circ Physiol. 2018;315(3): H448-H462. [3] CALCINOTTO A, KOHLI J, ZAGATO E, et al. Cellular Senescence: Aging, Cancer, and Injury. Physiol Rev. 2019;99(2):1047-1078. [4] COLLADO M, SERRANO M. The Power and the Promise of Oncogene-induced Senescence Markers. Nat Rev Cancer. 2006;6(6):472-476. [5] HERNANDEZ-SEGURA A, NEHME J, DEMARIA M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018;28(6):436-453. [6] MIJIT M, CARACCIOLO V, MELILLO A, et al. Role of P53 in the Regulation of Cellular Senescence. Biomolecules. 2020;10(3):420. [7] ZHU P, ZHANG C, GAO Y, et al. The Transcription Factor Slug Represses P16(Ink4a) and Regulates Murine Muscle Stem Cell Aging. Nat Commun. 2019;10(1):2568. [8] ZHU Y, TCHKONIA T, PIRTSKHALAVA T, et al. The Achilles’ Heel of Senescent Cells: from Transcriptome to Senolytic Drugs. Aging Cell. 2015;14(4):644-658. [9] WALASZCZYK A, DOOKUN E,REDGRAVE R, et al. Pharmacological Clearance of Senescent Cells Improves Survival and Recovery in Aged Mice Following Acute Myocardial Infarction. Aging Cell. 2019;18(3):e12945. [10] SHIMIZU I, MINAMINO T. Cellular Senescence in Cardiac Diseases. J Cardiol. 2019; 74(4):313-319. [11] WONG SQ, KUMAR AV, MILLS J, et al. Autophagy in Aging and Longevity. Hum Genet. 2020;139(3):277-290. [12] GUO F,LIU X,CAI H, et al. Autophagy in Neurodegenerative Diseases:Pathogenesis and Therapy. Brain Pathol. 2018;28(1):3-13. [13] LUO F, SANDHU AF, RUNGRATANAWANICH W, et al. Melatonin and Autophagy in Aging-Related Neurodegenerative Diseases. Int J Mol Sci. 2020;21(19):7174. [14] ALLAIRE M, RAUTOU PE, CODOGNO P, et al. Autophagy in Liver Diseases: Time for Translation? J Hepatol. 2019;70(5):985-998. [15] KE PY. Mitophagy in the Pathogenesis of Liver Diseases. Cells. 2020;9(4):831. [16] DU J, LIU Y, FU J. Autophagy and Heart Failure. Adv Exp Med Biol. 2020;1207:223-227. [17] CORSETTI G, PASINI E, ROMANO C, et al. How Can Malnutrition Affect Autophagy in Chronic Heart Failure? Focus and Perspectives. Int J Mol Sci. 2021;22(7):3332. [18] YOUNG A, NARITA R, FERREIRA M, et al. Autophagy Mediates the Mitotic Senescence Transition. Genes Dev. 2009;23(7):798-803. [19] KWON Y, KIM JW, JEOUNG JA, et al. Autophagy Is Pro-Senescence When Seen in Close-Up, but Anti-Senescence in Long-Shot. Mol Cells. 2017;40(9):607-612. [20] PULAKAT L, CHEN HH. Pro-Senescence and Anti-Senescence Mechanisms of Cardiovascular Aging: Cardiac MicroRNA Regulation of Longevity Drug-Induced Autophagy. Front Pharmacol. 2020;11:774. [21] ZHONG Y, WANG QJ, LI XT, et al. Distinct Regulation of Autophagic Activity by Atg14L and Rubicon Associated with Beclin 1-phosphatidylinositol-3-kinase Complex. Nature Cell Biol. 2009;11(4):468-476. [22] MATSUNAGA K, SAITOH T, TABATA K, et al. Two Beclin 1-binding Proteins, Atg14L and Rubicon,Reciprocally Regulate Autophagy at Different Stages. Nat Cell Biol. 2009;11(4):385-396. [23] KANG R, ZEH HJ, LOTZE MT, et al. The Beclin 1 Network Regulates Autophagy and Apoptosis. Cell Death and Differentiation. 2011;18(4):571-580. [24] SUN, QM, ZHANG J, FAN, WL, et al. The RUN Domain of Rubicon Is Important for hVps34 Binding, Lipid Kinase Inhibition, and Autophagy Suppression. Journal of Biological Chemistry. 2011;286(1):185-191. [25] SUN QM, ZHANG J, FAN WL, et al. Rubicon Controls Endosome Maturation as A Rab7 Effector. Proc Natl Acad Sci U S A. 2010;107(45):19338-19343. [26] NAKAMURA S, OBA M, SUZUKI M, et al. Suppression of Autophagic Activity by Rubicon is A Signature of Aging. Nat Commun. 2019;10(1):847. [27] SONG Z, AN L, YE Y, et al. Essential Role for UVRAG in Autophagy and Maintenance of Cardiac Function. Cardiovasc Res. 2014;101(1):48-56. [28] 来帅威,张沙沙,刘晓芸,等. 心脏细胞衰老与Uvrag基因的缺失[J]. 中国组织工程研究,2021,25(14):2241-2246. [29] ZI Z, SONG Z, ZHANG S, et al. Rubicon Deficiency Enhances Cardiac Autophagy and Protects Mice from Lipopolysaccharide-Induced Lethality and Reduction in Stroke Volume. J Cardiovasc Pharmacol. 2015;65(3):252-261. [30] THOMAS N, GURVICH C, KULKARNI J. Sex Differences in Aging and Associated Biomarkers. Adv Exp Med Biol. 2019;1178:57-76. [31] DODIG S, CEPELAK I, PAVIC I. Hallmarks of Senescence and Aging. Biochem Med (Zagreb). 2019;29(3):030501. [32] ZHANG J, CHENG P, PU K. Recent Advances of Molecular Optical Probes in Imaging of beta-Galactosidase. Bioconjug Chem. 2019;30(8):2089-2101. [33] LOPES-PACIENCIA S, SAINT-GERMAIN E, ROWELL MC, et al. The Senescence-associated Secretory Phenotype and its Regulation. Cytokine. 2019;117:15-22. [34] HOSHINO A, MITA Y, OKAWA Y, et al. Cytosolic P53 Inhibits Parkin-Mediated Mitophagy and Promotes Mitochondrial Dysfunction in The Mouse Heart. Nat Commun. 2013;4:2308. [35] REN X, CHEN L, XIE J, et al. Resveratrol Ameliorates Mitochondrial Elongation via Drp1/Parkin/PINK1 Signaling in Senescent-Like Cardiomyocytes. Oxid Med Cell Longev. 2017;2017:4175353. [36] MADEO F, TAVERNARAKIS N, KROEMER G. Can autophagy promote longevity? Nat Cell Biol. 2010;12(9):842-846. [37] FERNÁNDEZ ÁF, SEBTI S, WEI Y, et al. Disruption of The Beclin 1-BCL2 Autophagy Regulatory Complex Promotes Longevity in Mice. Nature. 2018;558(7708):136-140. [38] XU S, CAI Y, WEI Y. mTOR Signaling from Cellular Senescence to Organismal Aging. Aging Dis. 2014;5(4):263-273. [39] NISHIMURA A, SHIMAUCHI T, TANAKA T, et al. Hypoxia-Induced Interaction of Filamin with Drp1 Causes Mitochondrial Hyperfission-Associated Myocardial Senescence. Sci Signal. 2018;11(556):eaat5185. [40] ANDERSON R, LAGNADO A, MAGGIORANI D, et al. Length-Independent Telomere Damage Drives Post-Mitotic Cardiomyocyte Senescence. EMBO J. 2019;38(5): e100492. [41] LIU XY, ZHANG S, AN L, et al. Loss of Rubicon Ameliorates Doxorubicin-Induced Cardiotoxicity through Enhancement of Mitochondrial Quality. Int J Cardiol. 2019; 296:129-135. |
| [1] | Chen Chichi, Zhang Yu, He Jiachen, Shi Qin. Osteogenic differentiation of bone marrow mesenchymal stem cells in obese mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3846-3851. |
| [2] | Chen Sheng, Yang Yan, Ding Liang, Ye Chuanjin, Zhang Lei, Xia Shu, Li Huiling, Huang Xiaofeng. Establishment and identification of an immortalized oral cancer-related fibroblast line [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3808-3813. |
| [3] | Wei Wenyue, Wang Yuyin, Guo Minfang, Zhang Jing, Gu Qingfang, Song Lijuan, Chai Zhi, Yu Jiezhong, Ma Cungen. Fasudil inhibits neuronal apoptosis via regulating mitochondrial dynamics in APP/PS1 mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 232-238. |
| [4] | Mai Liping, He Guodong, Chen Shaoxian, Zhu Jiening, Hou Xinghua, Zhang Mengzhen, Li Xiaohong. Expression of aldehyde dehydrogenase 3B1 during human bone marrow mesenchymal stem cells senescence [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 40-44. |
| [5] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [6] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [7] | Liu Yang, Gong Yi, Fan Wei. Anti-hepatoma activity of targeted Pluronic F127/formononetin nanocomposite system in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 526-531. |
| [8] | Chen Dong, Jiang Xin. Effect of Kartogenin/Pluronic F127 micelles on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(34): 5473-5477. |
| [9] | Zhang Lishu, Liu Anqi, He Xiaoning, Jin Yan, Li Bei, Jin Fang. Alpl gene affects the therapeutic effect of bone marrow mesenchymal stem cells on ulcerative colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3970-3975. |
| [10] | Chen Siyu, Li Yannan, Xie Liying, Liu Siqi, Fan Yurong, Fang Changxing, Zhang Xin, Quan Jiayu, Zuo Lin. Thermosensitive chitosan-collagen composite hydrogel loaded with basic fibroblast growth factor retards ventricular remodeling after myocardial infarction in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2472-2478. |
| [11] | Chen Zhenyu, Zhang Xiaoning, Luo Yuxin, Liang Jianwei, Yan Chi. Evaluation of silk fibroin/curcumin composite film for promoting wound healing [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2554-2561. |
| [12] | Wang Donghui, Wu Xin, Sun Ningning, Zhang Han, Gao Jianfeng. Electroacupuncture intervention on the expression of synaptic plasticity-related proteins in the hippocampi of mice with radiation-induced brain injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(14): 2205-2210. |
| [13] | Lai Shuaiwei, Zhang Shasha, Liu Xiaoyun, Haniya Mazhar, Amber Naz, He Lin, Zhu Hongxin. Ultraviolet resistance-associated gene (Uvrag) deficiency promotes cellular senescence in the heart [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(14): 2241-2246. |
| [14] | Zhang Jingying, Sun Xiaolin, Geng Lixia. Therapeutic mechanism of adipose mesenchymal stem cells in mice with systemic sclerosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(13): 2011-2017. |
| [15] | Hua Hong, Xie Tongling, Hao Guiliang. Immunomodulatory effect of human adipose derived mesenchymal stem cells on skin transplantation between different mouse strains [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(1): 73-77. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||