Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (1): 34-42.doi: 10.12307/2026.507
Previous Articles Next Articles
Bone marrow mesenchymal stem cell nanovesicles fusion neutrophil apoptotic bodies promote skin wound healing in diabetic mice
Sun Zhanpeng1, Liu Sen1, Shi Ling1, Chen Kaiyuan1, Song Meichen1, Wu Yan1, Yu Jing2
- 1College of Life Sciences, Mudanjiang Medical University, Mudanjiang 157001, Heilongjiang Province, China; 2Department of Endocrinology, Hongqi Hospital, Mudanjiang Medical University, Mudanjiang 157001, Heilongjiang Province, China
-
Received:2024-10-31Accepted:2024-12-31Online:2026-01-08Published:2025-06-14 -
Contact:Yu Jing, MS, Associate chief physician, Department of Endocrinology, Hongqi Hospital, Mudanjiang Medical University, Mudanjiang 157001, Heilongjiang Province, China -
About author:Sun Zhanpeng, Research assistant, College of Life Sciences, Mudanjiang Medical University, Mudanjiang 157001, Heilongjiang Province, China -
Supported by:Mudanjiang City Guided Science and Technology Plan Project, No. HT2022JG125 (to WY)
CLC Number:
Cite this article
Sun Zhanpeng, Liu Sen, Shi Ling, Chen Kaiyuan, Song Meichen, Wu Yan, Yu Jing. Bone marrow mesenchymal stem cell nanovesicles fusion neutrophil apoptotic bodies promote skin wound healing in diabetic mice[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 34-42.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
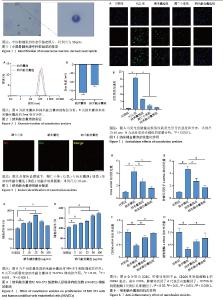
2.1 中性粒细胞的鉴定分析 如图1所示,在瑞氏染色血涂片中,中性粒细胞的胞体呈现圆形或类圆形,胞质颜色为透明或淡红色,内含许多分散的细小颗粒,呈浅红色或浅紫色;细胞核呈分叶状或杆状。 2.2 纳米融合囊泡的粒径和Zeta电位表征 纳米融合囊泡平均粒径与纳米囊泡相似并且粒径分布均匀,峰值粒径分别为(106.11±7.45) nm和(132.73±4.30) nm(图2A)。此外,纳米粒度及Zeta电位分析仪显示纳米融合囊泡带负电荷,Zeta电位为(-23.6±1.57) mV(图2B)。 2.3 纳米融合囊泡的膜融合验证 如图3所示,Dil膜染料(红色)标记的凋亡小体和Dio膜染料(绿色)标记的纳米囊泡通过微型挤出机处理后,形成具有良好荧光共定位的纳米融合囊泡(黄色),表明凋亡小体和纳米囊泡发生有效融合。 2.4 纳米融合囊泡对NIH-3T3细胞和HUVECs增殖的影响 如图4所示,0-100 μg/mL范围区间的纳米融合囊泡均能够促进NIH-3T3细胞和HUVECs的增殖。对于NIH-3T3细胞,25 μg/mL纳米融合囊泡具有最佳的促细胞增殖作用。对于HUVECs,细胞增殖活性随着纳米融合囊泡浓度的增加而不断增强。基于这一结果,选择25 μg/mL纳米融合囊泡进行后续实验研究。 2.5 纳米融合囊泡的抗氧化作用 将NIH-3T3细胞与H2O2(600 μmol/L)共同孵育模拟氧化应激环境。使用DCFH-DA作为荧光指示剂,以监测细胞内活性氧的生成水平。如图5所示,经H2O2处理后,细胞内观察到了高强度的荧光信号,这与细胞内活性氧水平显著增高的趋势紧密相关。与H2O2组相比较,纳米囊泡组和凋亡小体组荧光信号明显减弱,纳米融合囊泡组的活性氧荧光信号最弱,表明纳米融合囊泡可有效降低活性氧的生成。 2.6 纳米融合囊泡的体外抗炎效果 如图6所示,100 μg/L脂多糖刺激后巨噬细胞中促炎因子CD86和肿瘤坏死因子α的表达显著增加,而与脂多糖组相比较,纳米融合囊泡组促炎因子CD86和肿瘤坏死因子α的表达减少;另外,脂多糖刺激后巨噬细胞中抗炎因子CD206和精氨酸酶1的表达明显减少,而在纳米融合囊泡作用下CD206和精氨酸酶1的表达显著增加。"
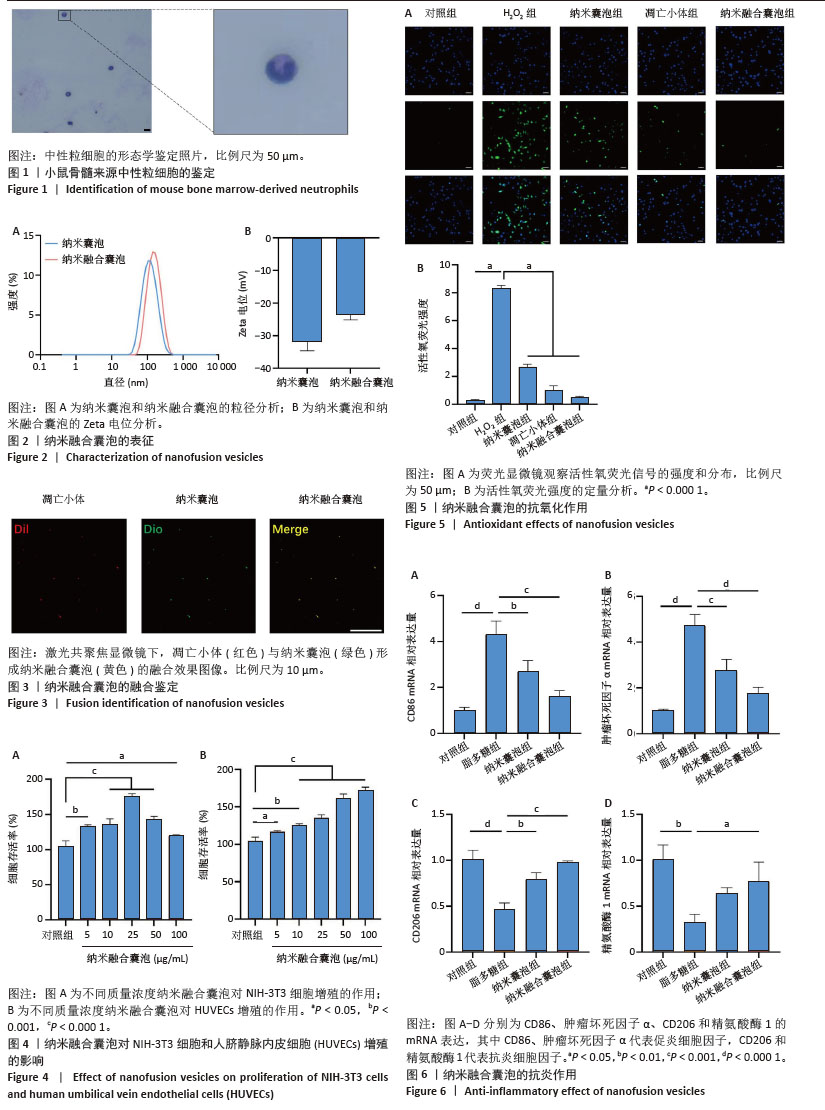
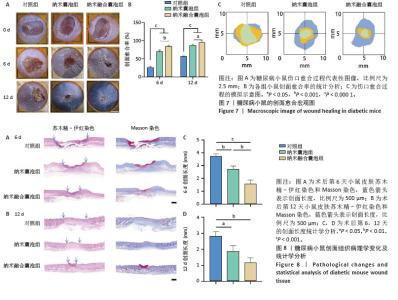
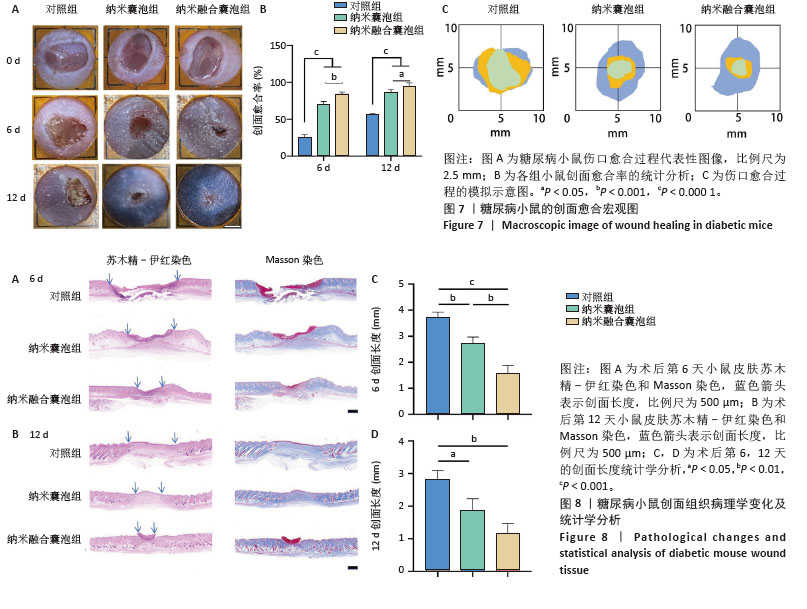
2.7 小鼠创面宏观愈合情况 2.7.1 实验动物数量分析 在实验过程中,所有参与实验的小鼠均未出现死亡现象,故将36只小鼠的数据纳入最终结果统计分析。 2.7.2 创面宏观愈合情况 使用小鼠全层糖尿病创面模型来评估纳米融合囊泡对创面愈合的影响。图7A直观展示了不同时间点各组小鼠伤口愈合的动态变化。图7B显示,在治疗第6天时,与对照组相比,纳米囊泡组和纳米融合囊泡组的创面面积显著缩小,创面愈合率分别为70.37%和84.26%。在治疗第12天时,各组创面面积进一步缩小,以纳米融合囊泡组的创面愈合速度最快,创面愈合率达到94.74%。图7C为第0,6,12天的伤口愈合过程模拟图。以上结果均表明,纳米融合囊泡显著促进了糖尿病小鼠的创面修复。 2.7.3 糖尿病小鼠的创面组织学染色分析 通过组织学染色分析来评估不同处理组的创面愈合效果。在治疗第6天时,苏木精-伊红染色显示(图8A,B),纳米囊泡组和纳米融合囊泡组的创面肉芽组织明显增厚,创面长度开始出现明显差异。其中,对照组的创面长度最长,为3.539 mm;而纳米融合囊泡组的创面长度最短,为1.31 mm(图8C)。在治疗第12天时,各组小鼠的创面长度进一步缩短,纳米囊泡组和纳米融合囊泡组的创面长度分别为1.54 mm和0.91 mm,相较于对照组的2.69 mm,创面愈合速度更快(图8D)。此外,纳米融合囊泡组可见大量皮肤附属器,而其他组未能观察到或仅有少量皮肤附属器。Masson染色显示(图8A,B),在治疗第6天时,相较于对照组,其余两组创面产生了更多的胶原沉积。在治疗第12天时,所有组相对于第6天显示出更多的胶原沉积,尤其是纳米融合囊泡组的胶原沉积最为显著。"
| [1] CEFALU WT, RIDDLE MC. More Evidence for a Prevention-Related Indication for Metformin: Let the Arguments Resume! Diabetes Care. 2019;42(4):499-501. [2] YU L, QIN J, XING J, et al. The mechanisms of exosomes in diabetic foot ulcers healing: a detailed review. J Mol Med (Berl). 2023;101(10): 1209-1228. [3] GENG X, QI Y, LIU X, et al. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater Adv. 2022;133:112613. [4] DENG H, LI B, SHEN Q, et al. Mechanisms of diabetic foot ulceration: A review. J Diabetes. 2023;15(4):299-312. [5] LI Z, ZHONG Q, YANG T, et al. The role of profilin-1 in endothelial cell injury induced by advanced glycation end products (AGEs). Cardiovasc Diabetol. 2013;12:141. [6] JU CC, LIU XX, LIU LH, et al. Epigenetic modification: A novel insight into diabetic wound healing. Heliyon. 2024;10(6):e28086. [7] LI F, LIU T, LIU X, et al. Ganoderma lucidum polysaccharide hydrogel accelerates diabetic wound healing by regulating macrophage polarization. Int J Biol Macromol. 2024;260(Pt 2):129682. [8] WU S, ZHOU Z, LI Y, et al. Advancements in diabetic foot ulcer research: Focus on mesenchymal stem cells and their exosomes. Heliyon. 2024; 10(17):e37031. [9] YU X, LIU P, LI Z, et al. Function and mechanism of mesenchymal stem cells in the healing of diabetic foot wounds. Front Endocrinol (Lausanne). 2023;14:1099310. [10] SUKMANA BI, MARGIANA R, ALMAJIDI YQ, et al. Supporting wound healing by mesenchymal stem cells (MSCs) therapy in combination with scaffold, hydrogel, and matrix; State of the art. Pathol Res Pract. 2023;248:154575. [11] PARK JS, SURYAPRAKASH S, LAO YH, et al. Engineering mesenchymal stem cells for regenerative medicine and drug delivery. Methods. 2015;84:3-16. [12] HUANG L, WU E, LIAO J, et al. Research Advances of Engineered Exosomes as Drug Delivery Carrier. ACS Omega. 2023;8(46):43374-43387. [13] MA YN, HU X, KARAKO K, et al. Exploring the multiple therapeutic mechanisms and challenges of mesenchymal stem cell-derived exosomes in Alzheimer’s disease. Biosci Trends. 2024;18(5):413-430. [14] WANG X, HU S, LI J, et al. Extruded Mesenchymal Stem Cell Nanovesicles Are Equally Potent to Natural Extracellular Vesicles in Cardiac Repair. ACS Appl Mater Interfaces. 2021;13(47):55767-55779. [15] JIAO Y, ZHANG T, ZHANG C, et al. Exosomal miR-30d-5p of neutrophils induces M1 macrophage polarization and primes macrophage pyroptosis in sepsis-related acute lung injury. Crit Care. 2021;25(1):356. [16] BRAZA MS, CONDE P, GARCIA M, et al. Neutrophil derived CSF1 induces macrophage polarization and promotes transplantation tolerance. Am J Transplant. 2018;18(5):1247-1255. [17] AKKUS G, SERT M. Diabetic foot ulcers: A devastating complication of diabetes mellitus continues non-stop in spite of new medical treatment modalities. World J Diabetes. 2022;13(12):1106-1121. [18] SYED MH, SALATA K, HUSSAIN MA, et al. The economic burden of inpatient diabetic foot ulcers in Toronto, Canada. Vascular. 2020;28(5): 520-529. [19] QIN W, WU Y, LIU J, et al. A Comprehensive Review of the Application of Nanoparticles in Diabetic Wound Healing: Therapeutic Potential and Future Perspectives. Int J Nanomedicine. 2022;17:6007-6029. [20] LI J, WU Z, ZHAO L, et al. The heterogeneity of mesenchymal stem cells: an important issue to be addressed in cell therapy. Stem Cell Res Ther. 2023;14(1):381. [21] SHI L, ZHOU Y, YIN Y, et al. Advancing Tissue Damage Repair in Geriatric Diseases: Prospects of Combining Stem Cell-Derived Exosomes with Hydrogels. Int J Nanomedicine. 2024;19:3773-3804. [22] MONDAL J, PILLARISETTI S, JUNNUTHULA V, et al. Hybrid exosomes, exosome-like nanovesicles and engineered exosomes for therapeutic applications. J Control Release. 2023;353:1127-1149. [23] CHEN J, LI P, ZHANG T, et al. Review on Strategies and Technologies for Exosome Isolation and Purification. Front Bioeng Biotechnol. 2022;9:811971. [24] CABRINI M, NAHMOD K, GEFFNER J. New insights into the mechanisms controlling neutrophil survival. Curr Opin Hematol. 2010;17(1):31-35. [25] SKENDROS P, MITROULIS I, RITIS K. Autophagy in Neutrophils: From Granulopoiesis to Neutrophil Extracellular Traps. Front Cell Dev Biol. 2018; 6:109. [26] DORAN AC, YURDAGUL A JR, TABAS I. Efferocytosis in health and disease. Nat Rev Immunol. 2020;20(4):254-267. [27] LIEBOLD I, AL JAWAZNEH A, CASAR C, et al. Apoptotic cell identity induces distinct functional responses to IL-4 in efferocytic macrophages. Science. 2024;384(6691):eabo7027. [28] CAMPBELL EL, KAO DJ, COLGAN SP. Neutrophils and the inflammatory tissue microenvironment in the mucosa. Immunol Rev. 2016;273(1):112-120. [29] KIM DY, KANG YH, KANG MK. Umbelliferone alleviates impaired wound healing and skin barrier dysfunction in high glucose-exposed dermal fibroblasts and diabetic skins. J Mol Med (Berl). 2024;102(12):1457-1470. [30] 施芳婷,沈佳琪,仵敏娟.糖尿病难愈性创面的发病机制研究进展[J].生命科学,2024,36(4):509-516. [31] ZAKERI A, KHASEB S, AKHAVAN RAHNAMA M, et al. Exosomes derived from mesenchymal stem cells: A promising cell-free therapeutic tool for cutaneous wound healing. Biochimie. 2023;209:73-84. [32] ABDULMALEK OAAY, HUSAIN KH, ALKHALIFA HKAA, et al. Therapeutic Applications of Stem Cell-Derived Exosomes. Int J Mol Sci. 2024;25(6):3562. [33] ARIAS-CALDERÓN M, CASAS M, BALANTA-MELO J, et al. Fibroblast growth factor 21 is expressed and secreted from skeletal muscle following electrical stimulation via extracellular ATP activation of the PI3K/Akt/mTOR signaling pathway. Front Endocrinol (Lausanne). 2023;14:1059020. [34] ZHANG B, BI Y, WANG K, et al. Stem Cell-Derived Extracellular Vesicles: Promising Therapeutic Opportunities for Diabetic Wound Healing. Int J Nanomedicine. 2024;19:4357-4375. [35] CHEN P, ZHENG L, WANG Y, et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9(9):2439-2459. [36] ZHANG Y, PAN Y, LIU Y, et al. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate regenerative wound healing via transforming growth factor-β receptor inhibition. Stem Cell Res Ther. 2021;12(1):434. [37] 苏梦,王昕,张津,等.纳米细胞囊泡负载姜黄素促进糖尿病小鼠创面的愈合[J].中国组织工程研究,2023,27(12):1877-1883. [38] FENG J, YAO Y, WANG Q, et al. Exosomes: Potential key players towards novel therapeutic options in diabetic wounds. Biomed Pharmacother. 2023;166:115297. [39] ZAMANIAN MY, ALSAAB HO, GOLMOHAMMADI M, et al. NF-κB pathway as a molecular target for curcumin in diabetes mellitus treatment: Focusing on oxidative stress and inflammation. Cell Biochem Funct. 2024;42(4):e4030. [40] WU Y, SU M, ZHANG S, et al. A mesenchymal stem cell-derived nanovesicle-biopotentiated bovine serum albumin-bridged gelatin hydrogel for enhanced diabetic wound therapy. Mater Design. 2023;230:111960. [41] BURGESS JL, WYANT WA, ABDO ABUJAMRA B, et al. Diabetic Wound-Healing Science. Medicina (Kaunas). 2021;57(10):1072. [42] BAO L, DOU G, TIAN R, et al. Engineered neutrophil apoptotic bodies ameliorate myocardial infarction by promoting macrophage efferocytosis and inflammation resolution. Bioact Mater. 2021;9:183-197. [43] DHANDHI S, YESHNA, VISHAL, et al. The interplay of skin architecture and cellular dynamics in wound healing: Insights and innovations in care strategies. Tissue Cell. 2024;91:102578. |
| [1] | Wen Fan, Xiang Yang, Zhu Huan, Tuo Yanfang, Li Feng. Exercise improves microvascular function in patients with type 2 diabetes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1225-1235. |
| [2] | Wen Xiaolong, Weng Xiquan, Feng Yao, Cao Wenyan, Liu Yuqian, Wang Haitao. Effects of inflammation on serum hepcidin and iron metabolism related parameters in patients with type 2 diabetes mellitus: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1294-1301. |
| [3] | Yan Chengbo, Luo Qiuchi, Fan Jiabing, Gu Yeting, Deng Qian, Zhang Junmei. Effect of type 2 diabetes mellitus on orthodontic tooth movement and bone microstructure parameters on the tension side in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 824-831. |
| [4] | Zhang Tingting, Li Yalong, Yue Haodi, Li Yanjun, Geng Xiwen, Zhang Yuwei, Liu Xiaozhuan. Protection of exosomes derived from bone marrow mesenchymal stem cells of different mouse ages on radiation-induced lung injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 1-9. |
| [5] | Sun Huiwen, Guo Qiangqiang, Wang Wei, Wu Jie, Xi Kun, Gu Yong. Engineered stem cell bionic periosteum coordinates immune inflammation and vascularization to promote bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 21-33. |
| [6] | Chen Qiheng, Weng Tujun, Peng Jiang. Effect of dimethylglyoxal glycine on osteogenic, adipogenesis differentiation, and mitophagy of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 50-57. |
| [7] | Yu Daiyao, Shi Ping, Yang Lan, Li Zhishu, Lu Yongping. Advances and application of neutrophil extracellular traps and activated platelets in lung cancer research [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 229-237. |
| [8] | Su Xiaoyang, Chen Wenting, Fu Yidan, Zhao Yan, Lan Danfeng, Yang Qiuping. Correlation between Mer receptor tyrosine kinase and diabetic peripheral neuropathy in Sprague-Dawley rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1593-1599. |
| [9] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [10] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [11] | Yu Ting, Lyu Dongmei, Deng Hao, Sun Tao, Cheng Qian. Icariin pretreatment enhances effect of human periodontal stem cells on M1-type macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1328-1335. |
| [12] | Yang Zhihang, Sun Zuyan, Huang Wenliang, Wan Yu, Chen Shida, Deng Jiang. Nerve growth factor promotes chondrogenic differentiation and inhibits hypertrophic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1336-1342. |
| [13] | Sun Xianjuan, Wang Qiuhua, Zhang Jinyi, Yang Yangyang, Wang Wenshuang, Zhang Xiaoqing. Adhesion, proliferation, and vascular smooth muscle differentiation of bone marrow mesenchymal stem cells on different electrospinning membranes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 661-669. |
| [14] | Dong Meilin, Du Haiyu, Liu Yuan. Quercetin-loaded carboxymethyl chitosan hydrogel promotes wound healing in diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 692-699. |
| [15] | Ge Xiao, Zhao Zhuangzhuang, Guo Shuyu, Xu Rongyao. HOXA10 gene-modified bone marrow mesenchymal stem cells promote bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7701-7708. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||