Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (34): 7261-7268.doi: 10.12307/2025.894
Comparison of Mg-Li-Gd alloy and stainless steel intramedullary nail for fixation of femoral annular hemi-defects in rats
Wang Jingshuai1, Zhang Xiaotong2, Zhang Yange1, Wan Zedong1, Kong Lingwei1, Cao Haiying1, Jin Yu1
- 1Affiliated Hospital of Chengde Medical University, Chengde 067000, Hebei Province, China; 2Chengde Medical University, Chengde 067000, Hebei Province, China
-
Received:2024-08-12Accepted:2024-10-16Online:2025-12-08Published:2025-01-17 -
Contact:Jin Yu, Chief physician, Affiliated Hospital of Chengde Medical University, Chengde 067000, Hebei Province, China -
About author:Wang Jingshuai, Master candidate, Attending physician, Affiliated Hospital of Chengde Medical University, Chengde 067000, Hebei Province, China -
Supported by:Natural Science Foundation of Hebei Province, No. C2022203003
CLC Number:
Cite this article
Wang Jingshuai, Zhang Xiaotong, Zhang Yange, Wan Zedong, Kong Lingwei, Cao Haiying, Jin Yu. Comparison of Mg-Li-Gd alloy and stainless steel intramedullary nail for fixation of femoral annular hemi-defects in rats[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7261-7268.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
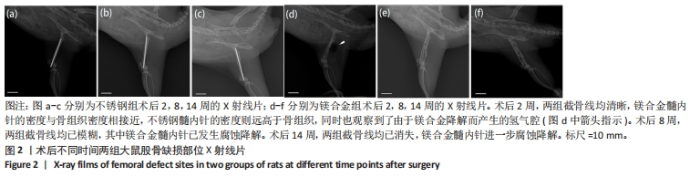
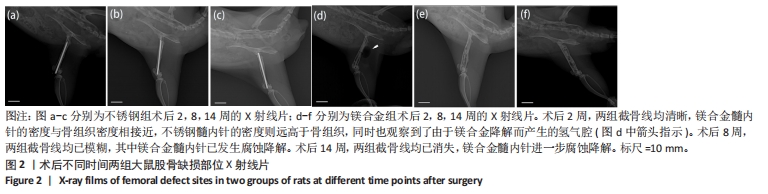
2.1 实验动物数量分析 实验选用28只SD大鼠,实验过程中有2只大鼠(不锈钢组与镁合金组各1只)因麻醉过量死亡,最后进入结果分析26只,不锈钢组与镁合金组各13只。 2.2 大鼠切口愈合与肢体活动情况 术后可见大鼠切口愈合情况良好,无感染或红肿,周围皮肤无肿胀、淤血或异常变色,右下肢无明显的错位或移位,关节活动度正常,无僵硬或异常反应。 2.3 各组大鼠右侧股骨X射线片检查结果 术后不同时间两组大鼠右侧股骨X射线片,见图2。术后2周,两组截骨线均清晰,髓内针直径与骨髓腔直径匹配良好,其中镁合金髓内针的密度与骨组织密度相接近,不锈钢髓内针的密度则远高于骨组织,同时也观察到了由于镁合金降解而产生的氢气腔。术后8周,两组截骨线均已模糊,无明显差别,其中镁合金髓内针已发生腐蚀降解,氢气腔消失。术后14周,两组截骨线均已消失,镁合金髓内针进一步腐蚀降解,未观察到氢气腔。"
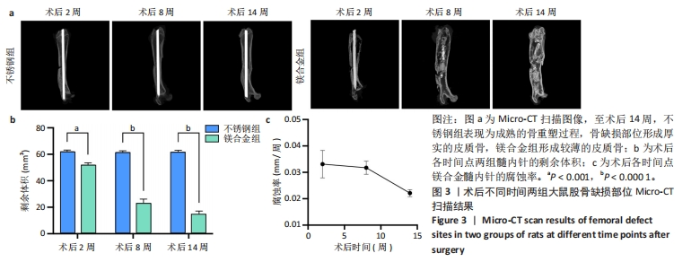
2.4 各组大鼠右侧股骨Micro-CT扫描结果 术后不同时间两组大鼠右侧股骨Micro-CT扫描图像,见图3a。术后2周,不锈钢组呈现骨折愈合的早期阶段,骨痂开始形成;镁合金组表现出类似的早期骨愈合迹象,骨痂也开始形成。术后8周,不锈钢组进入骨痂重塑阶段,骨组织逐步填充骨缺损部位,形成较为致密的骨结构,股骨直径保持较为稳定;镁合金组呈现明显的骨痂增生,股骨中段直径显著大于不锈钢组,骨痂结构相对较为疏松。术后14周,不锈钢组呈现较为成熟的骨重塑过程,骨组织趋于稳固,骨痂完全填补了骨缺损部位,形成厚实的皮质骨;镁合金组股骨体积进一步增加,骨直径显著大于不锈钢组,但皮质骨较薄,骨缺损处依然存在纤维性组织连接的痕迹,而非完全成熟的骨组织。 随着时间推移,镁合金髓内针逐渐降解。术后2周,镁合金组髓内针体积减少明显,不锈钢组髓内针体积无显著变化;术后8-14周,镁合金组髓内针残留体积显著减少(图3b)。根据腐蚀率曲线显示,镁合金髓内针降解速率在前8周较快,而在后续时间段有所减缓(图3c)。"
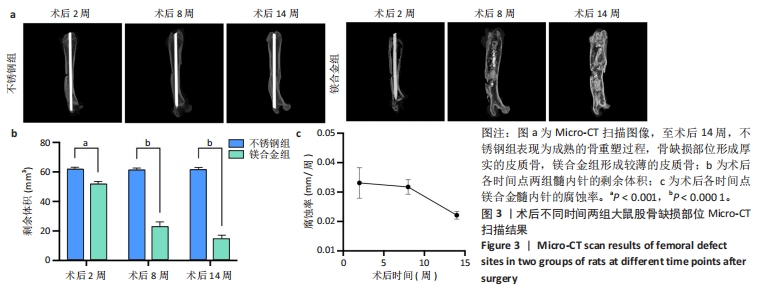
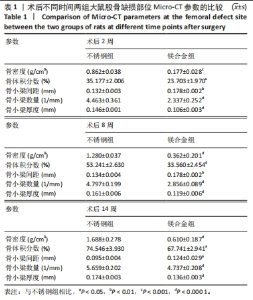
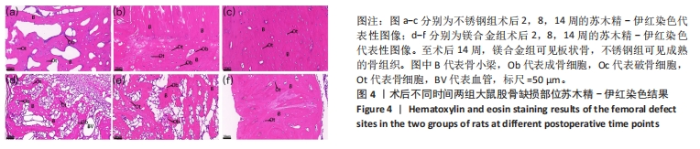
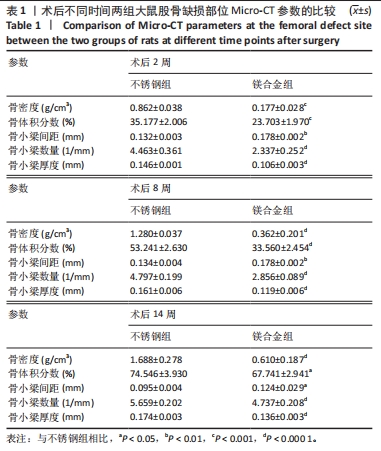
术后不同时间两组大鼠股骨缺损部位Micro-CT参数比较,见表1。相同时间下,镁合金组骨密度、骨体积分数、骨小梁数量、骨小梁厚度均低于不锈钢组(P < 0.05,P < 0.001,P < 0.000 1),骨小梁间距均大于不锈钢组(P < 0.05,P < 0.01)。 2.5 各组大鼠右侧股骨苏木精-伊红染色结果 术后不同时间各组大鼠右侧股骨骨缺损部位苏木精-伊红染色结果,见图4。术后2周,镁合金组可见大量的成骨细胞、骨细胞及少量的破骨细胞、骨小梁结构,不锈钢组可见相对较少的成骨细胞和骨细胞,骨小梁结构相较于镁合金组未见明显变化。术后8周,镁合金组可见大量的成骨细胞、骨细胞,可见成熟的排列较规则的骨小梁结构,骨小梁周围有活跃的成骨细胞;不锈钢组可见大量的骨细胞及红染的板状骨。术后14周,镁合金组可见红染的板状骨,不锈钢组可见经骨重塑的成熟骨组织。"
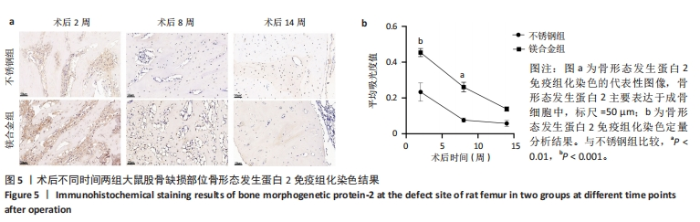
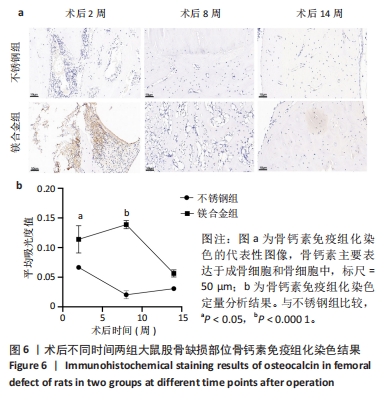
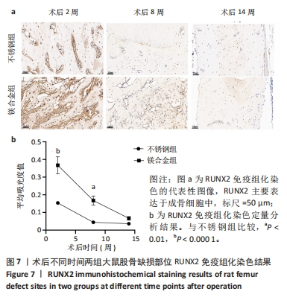
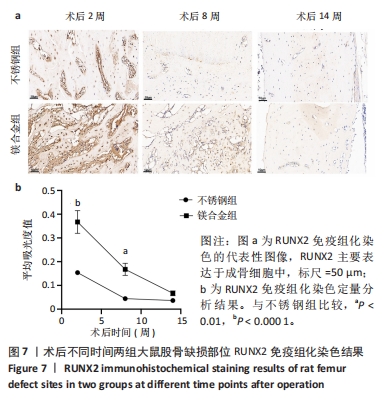
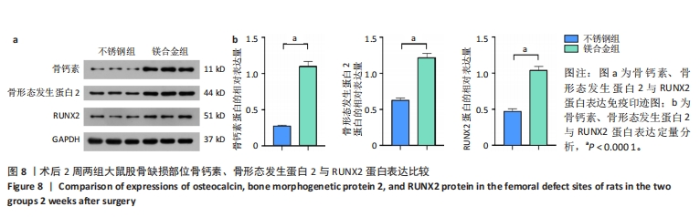
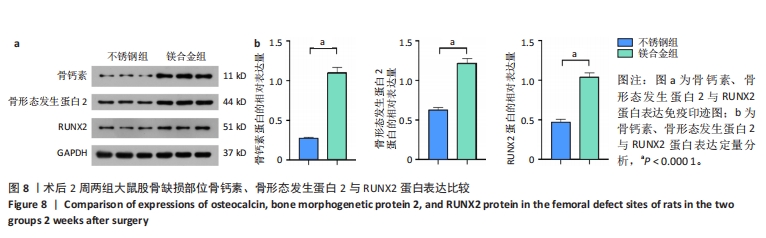
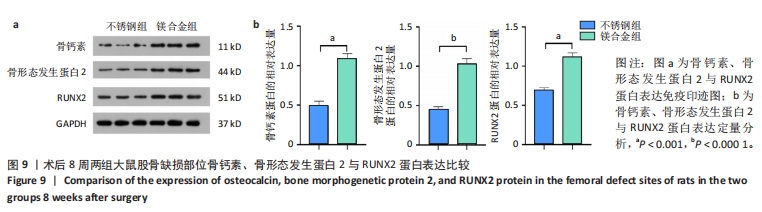
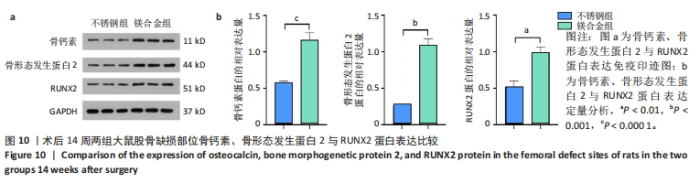
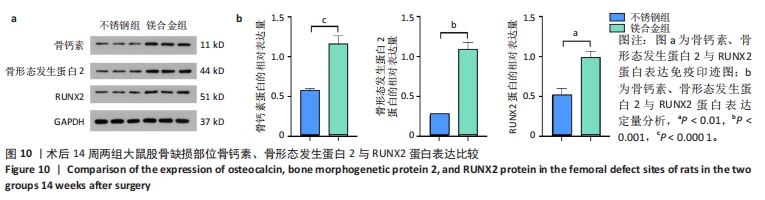
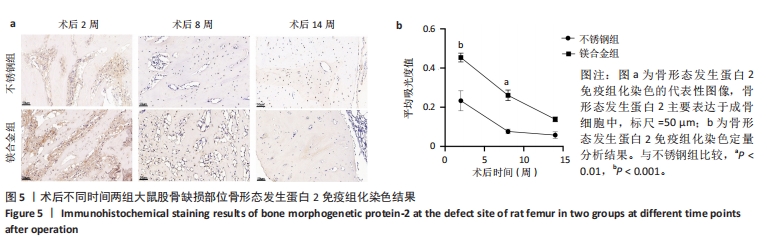
2.6 各组大鼠右侧股骨免疫组化染色结果 术后不同时间两组大鼠股骨缺损部位骨形态发生蛋白2免疫组化染色结果,见图5。骨形态发生蛋白2主要表达于成骨细胞中,定量分析结果显示镁合金组术后2,8周的骨形态发生蛋白2表达高于不锈钢组(P < 0.001,P < 0.01)。术后不同时间两组大鼠股骨缺损部位骨钙素免疫组化染色结果,见图6。骨钙素主要表达于成骨细胞和骨细胞中,定量分析结果显示镁合金组术后2,8周的骨钙素表达高于不锈钢组(P < 0.05,P < 0.000 1)。术后不同时间两组大鼠股骨缺损部位RUNX2免疫组化染色结果,见图7。RUNX2主要表达于成骨细胞中,定量分析结果显示镁合金组术后2,8周的RUNX2表达高于不锈钢组(P < 0.000 1,P < 0.01)。"
| [1] FOESSL I, DIMAI HP, OBERMAYER-PIETSCH B. Long-term and sequential treatment for osteoporosis. Nat Rev Endocrinol. 2023;19(9):520-533. [2] AYERS C, KANSAGARA D, LAZUR B, et al. Effectiveness and Safety of Treatments to Prevent Fractures in People With Low Bone Mass or Primary Osteoporosis: A Living Systematic Review and Network Meta-analysis for the American College of Physicians. Ann Intern Med. 2023; 176(2):182-195. [3] MIRAMINI S, ZHANG L, RICHARDSON M, et al. Influence of fracture geometry on bone healing under locking plate fixations: A comparison between oblique and transverse tibial fractures. Med Eng Phys. 2016; 38(10):1100-1108. [4] FAN L, CHEN S, YANG M, et al. Metallic materials for bone repair. Adv Healthc Mater. 2024;13(3):e2302132. [5] AMUKARIMI S, MOZAFARI M. Biodegradable magnesium-based biomaterials: An overview of challenges and opportunities. MedComm (2020). 2021;2(2):123-144. [6] INNES MB, ATWATER AR. Orthopedic Implant Hypersensitivity Reactions: Concepts and Controversies. Dermatol Clin. 2020;38(3):361-369. [7] ANTONIAC I, MICULESCU M, MĂNESCU PĂLTÂNEA V, et al. Magnesium-Based Alloys Used in Orthopedic Surgery. Materials (Basel). 2022; 15(3):1148. [8] DE RUITER L, RANKIN K, BROWNE M, et al. Decreased stress shielding with a PEEK femoral total knee prosthesis measured in validated computational models. J Biomech. 2021;118:110270. [9] CHAKRABORTY BANERJEE P, AL-SAADI S, CHOUDHARY L, et al. Magnesium Implants: Prospects and Challenges. Materials (Basel). 2019;12(1):136. [10] NASR AZADANI M, ZAHEDI A, BOWOTO OK, et al. A review of current challenges and prospects of magnesium and its alloy for bone implant applications. Prog Biomater. 2022;11(1):1-26. [11] KUAH KX, WIJESINGHE S, BLACKWOOD DJ. Toward understanding in vivo corrosion: Influence of interfacial hydrogen gas build-up on degradation of magnesium alloy implants. J Biomed Mater Res A. 2023;111(1):60-70. [12] FAN L, CHEN S, YANG M, et al. Metallic Materials for Bone Repair. Adv Healthc Mater. 2024;13(3):e2302132. [13] LI J, ZHOU P, WANG L, et al. Investigation of Mg-xLi-Zn alloys for potential application of biodegradable bone implant materials. J Mater Sci Mater Med. 2021;32(4):43. [14] HANKE L, JESSEN LK, WEISHEIT F, et al. Structural characterisation and degradation of Mg-Li thin films for biodegradable implants. Sci Rep. 2023;13(1):12572. [15] SARANYA K, KALAIYARASAN M, AGILAN P, et al. Biofunctionalization of Mg implants with gadolinium coating for bone regeneration. Surf Interfaces. 2022;31:101948. [16] YU Z, HUANG Y, LIU L, et al. New strategy to solve the ambient strength-ductility dilemma in precipitation-strengthened Mg-Gd alloys via Li addition. Scr Mater. 2022;220:114901. [17] GUAN X, XIONG M, ZENG F, et al. Enhancement of osteogenesis and biodegradation control by brushite coating on Mg-Nd-Zn-Zr alloy for mandibular bone repair. ACS Appl Mater Interfaces. 2014;6(23):21525-21533. [18] KUTIKOV AB, SKELLY JD, AYERS DC, et al. Templated repair of long bone defects in rats with bioactive spiral-wrapped electrospun amphiphilic polymer/hydroxyapatite scaffolds. ACS Appl Mater Interfaces. 2015; 7(8):4890-4901. [19] WANG G, JIANG W, MO S, et al. Nonleaching Antibacterial Concept Demonstrated by In Situ Construction of 2D Nanoflakes on Magnesium. Adv Sci (Weinh). 2020;7(1):1902089. [20] GUO J, TIAN S, WANG Z, et al. Hydrogen saline water accelerates fracture healing by suppressing autophagy in ovariectomized rats. Front Endocrinol (Lausanne). 2022;13:962303. [21] KRAUS T, FISCHERAUER SF, HÄNZI AC, et al. Magnesium alloys for temporary implants in osteosynthesis: in vivo studies of their degradation and interaction with bone. Acta Biomater. 2012;8(3): 1230-1238. [22] HAN HS, JUN I, SEOK HK, et al. Biodegradable magnesium alloys promote angio-osteogenesis to enhance bone repair. Adv Sci (Weinh). 2020;7(15):2000800. [23] HUANG TB, LI YZ, YU K, et al. Effect of the Wnt signal-RANKL/OPG axis on the enhanced osteogenic integration of a lithium incorporated surface. Biomater Sci. 2019;7(3):1101-1116. [24] LIU W, CHEN D, JIANG G, et al. A lithium-containing nanoporous coating on entangled titanium scaffold can enhance osseointegration through Wnt/β-catenin pathway. Nanomed. 2018;14(1):153-164. [25] ZHU DY, LU B, YIN JH, et al. Gadolinium-doped bioglass scaffolds promote osteogenic differentiation of hBMSC via the Akt/GSK3β pathway and facilitate bone repair in vivo. Int J Nanomedicine. 2019;14:1085-1100. [26] SONG X, LIU X, MA Y, et al. Synthesis of Ce/Gd@HA/PLGA Scaffolds Contributing to Bone Repair and MRI Enhancement. Front Bioeng Biotechnol. 2022;10:834226. [27] LEIDI M, DELLERA F, MARIOTTI M, et al. High magnesium inhibits human osteoblast differentiation in vitro. Magnes Res. 2011;24(1):1-6. [28] ZHANG L, YANG C, LI J, et al. High extracellular magnesium inhibits mineralized matrix deposition and modulates intracellular calcium signaling in human bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2014;450(4):1390-1395. [29] FLORENCIO-SILVA R, SASSO GR, SASSO-CERRI E, et al. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed Res Int. 2015;2015:421746. [30] TSAO YT, SHIH YY, LIU YA, et al. Knockdown of SLC41A1 magnesium transporter promotes mineralization and attenuates magnesium inhibition during osteogenesis of mesenchymal stromal cells. Stem Cell Res Ther. 2017;8(1):39. [31] NAKATANI S, MANO H, RYANGHYOK IM, et al. Excess magnesium inhibits excess calcium-induced matrix-mineralization and production of matrix gla protein (MGP) by ATDC5 cells. Biochem Biophys Res Commun. 2006;348(3):1157-1162. [32] HARTJEN P, WEGNER N, AHMADI P, et al. Toward Tailoring the Degradation Rate of Magnesium-Based Biomaterials for Various Medical Applications: Assessing Corrosion, Cytocompatibility and Immunological Effects. Int J Mol Sci. 2021;22(2):971. [33] VIRTANEN S. Biodegradable Mg and Mg alloys: Corrosion and biocompatibility. Mater Sci Eng B. 2011;176(20):1600-1608. [34] GYÖRGYEY Á, UNGVÁRI K, KECSKEMÉTI G, et al. Attachment and proliferation of human osteoblast-like cells (MG-63) on laser-ablated titanium implant material. Mater Sci Eng C Mater Biol Appl. 2013; 33(7):4251-4259. [35] ANAND N, MEHROTRA N, PAL K. Biodegradable implant application: Electrodeposition of HA/TiO2/ZrO2 coating onto Zn-composite substrates. J Mech Behav Biomed Mater. 2023;146:106073. |
| [1] | Liu Haoyang, Xie Qiang, Shen Mengran, Ren Yansong, Ma Jinhui, Wang Bailiang, Yue Debo, Wang Weiguo . Application, research hotspots, and shortcomings of degradable zinc-based alloys in bone defect repair and reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 839-845. |
| [2] | Zhao Shuai, Li Dongyao, Wei Suiyan, Cao Yijing, Xu Yan, Xu Guoqiang. Biocompatibility of poly(vinylidene fluoride) piezoelectric bionic periosteum prepared by electrospinning [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 730-737. |
| [3] | Zhou Yang, Liu Kexin, Wang Deli, Sun Zhang. Regenerative effects of engineered extracellular vesicles on repairing bone defects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7839-7847. |
| [4] | Ma Yucong, Ouyang Zhengzheng, Liu Xiaojie, Yang Sifei. Tracking of research trends and hotspots in medical magnesium alloy materials [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7470-7480. |
| [5] | Ren Bo, Tang Yongliang, Li Ni, Liu Bangding. Thermosensitive antibacterial hydrogel for treatment of infected bone defects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7269-7277. |
| [6] | Wu Ziwei, Luo Yicai, Wei Yinge, Liao Hongbing. Application of poly(lactic-co-glycolic acid) copolymer in stomatology [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7393-7404. |
| [7] | Deng Guanghui, Xiang Wei, Su Qifan, Chen Xiaoyu, Wang Liangwei, Wan Zhihong, Wu Jiaqi, Chen Xiaojun. Preparation of osteoporotic femoral condylar bone defect model in rabbits and its critical value [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6426-6433. |
| [8] | Li Shuyuan, Yang Dawen, Zeng Zhanpeng, Cai Qunbin, Zhang Jingtao, Zhou Qishi. Application of induced membrane technique for repairing critical-sized bone defects: advantages and future development [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 6083-6093. |
| [9] | Shen Zhen, Huang Ziyue, He Zhijuan, Wang Yiting, Chen Qigang, Geng Chunmei, Huang Yajing, Wu Zugui. Dynamic expression of H-type vessels coupled with bone repair effect in bone induced membrane for massive bone defects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 5950-5956. |
| [10] | Lu Yuzheng, Xiong Yingjie, Shan Yanbo, Ye Jianting, Wu Yanbin, Song Jipeng, Zhang Yao, Lin Wancheng, Weng Qirui, Cheng Xuan, Meng Haoye, Xu Wenjing, Peng Jiang, Ding Lixiang. Effect of silicate bioactive glass fiber on properties of calcium phosphate bone cement [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 5994-6002. |
| [11] | Mutalipu·Silamujiang, Yusufu·Reheman, Ren Zheng, Alimujiang·Yusufu, Ran Jian, Wang Jian. Finite element analysis of three internal fixation methods for treating Pauwels III femoral neck fractures with anterior medial bone defects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(27): 5721-5727. |
| [12] | Li Guangzhao, Pei Xibo, Wang Jian. Application of nanoparticles in gene modification therapy for bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(22): 4771-4783. |
| [13] | Jiang Zhixiu, Ji Yuchen, Liu Danyu, Cao Yilin, Jiang Tingting, Song Yihan, Wang Lei, Wang Xinyu. Biomechanical properties of Gyroid structured titanium bionic bone scaffolds for repairing segmental mandibular defects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(22): 4621-4628. |
| [14] | Ji Mingyi, Ji Xinyi, Xu Junfeng. Mechanism by which melatonin promotes bone regeneration and its application in oral implantation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3868-3876. |
| [15] | Li Yonghang, Li Wenming, Yan Caiping, Wang Xingkuan, Xiang Chao, Zhang Yuan, Jiang Ke, Chen Lu. Critical bone defect repaired with anti-fibrosis and “H”-type core-shell bionic scaffold [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3420-3431. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||