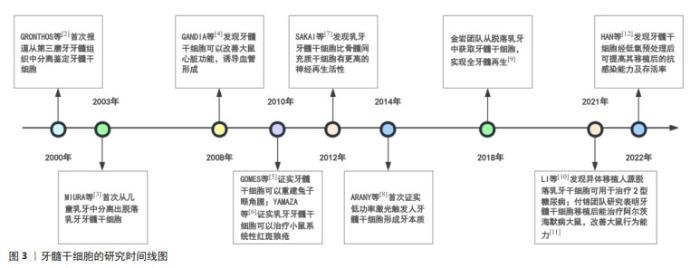
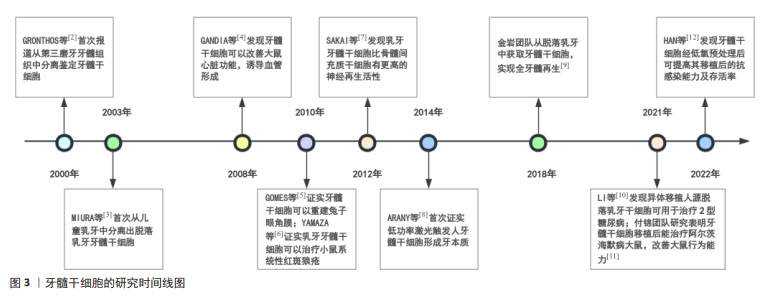
Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (7): 1531-1540.doi: 10.12307/2024.743
Physical factors and action mechanisms affecting osteogenic/odontogenic differentiation of dental pulp stem cells
Sun Yuting, Wu Jiayuan, Zhang Jian
- School of Stomatology, Zunyi Medical University, Zunyi 563000, Guizhou Province, China
-
Received:2023-09-27Accepted:2023-12-20Online:2025-03-08Published:2024-06-28 -
Contact:Zhang Jian, Associate professor, Master’s supervisor, School of Stomatology, Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
About author:Sun Yuting, Master candidate, School of Stomatology, Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
Supported by:Science and Technology Fund Project of Guizhou Provincial Health Commission, No. gzwjkj2020-1-163 (to WJY); Oral Infectious and Malignant Disease Etiology and Prevention Innovation Team Project of Affiliated Stomatological Hospital of Zunyi Medical University, No. Zunyi Kehe HZ(2020)293 (to WJY); Science and Technology Program of Guizhou Province, No. ZK[2022]-Normal638 (to WJY)
CLC Number:
Cite this article
Sun Yuting, Wu Jiayuan, Zhang Jian. Physical factors and action mechanisms affecting osteogenic/odontogenic differentiation of dental pulp stem cells[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1531-1540.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

2.2 影响牙髓干细胞成骨/成牙本质分化的相关物理因素及作用机制 2.2.1 低氧 氧浓度是细胞培养的关键环境因素,不同氧张力下细胞有不同的细胞表型以及增殖分化潜能。细胞正常的生理功能需要ATP提供能量,在有氧和无氧条件下分别通过氧化磷酸化和糖酵解产生ATP,在低氧状态时,细胞代谢转向无氧糖酵解产生ATP。正常情况下空气中的氧浓度约为21%,人体细胞微环境中氧浓度要低于空气中的氧浓度,不同组织细胞生理活动的需氧量不一致,人类胚胎早期接触的氧浓度为1%-9%,说明低氧对生物体的发育存在着重要意义,牙髓干细胞是一种特殊的间充质干细胞,牙髓是位于髓腔内的疏松结缔组织,因其被坚硬的牙本质包围,氧气只能通过根管内的脉管系统到达牙髓组织,因此使细胞长期处于一个低氧状态[13]。建立细胞低氧模型是从细胞及分子水平研究相关疾病的重要方法,通过降低培养环境的氧分压造成细胞缺氧是物理性缺氧法,而化学性缺氧法则是加入氰化物及氧化钴等某些化学物质,因其对细胞可能有损伤作用且增加了混杂因素,所以慎重使用,物理性缺氧法更接近于牙髓细胞本身的缺氧状态,所以也是目前常用的低氧培养方法。低氧对牙髓干细胞成骨/成牙本质分化的影响文献研究不够全面且存在一定争议,不同的氧体积分数以及处理时间等均会对结果产生影响,但以促进牙髓干细胞分化为主,关于氧浓度设定为多少才是影响牙髓干细胞成骨/成牙本质分化的最佳条件目前尚无定论。 低氧环境对牙髓干细胞生物学特性的影响涉及多因子多通路,其中低氧诱导因子1α是介导细胞对缺氧微环境适应的关键转录因子,可调控细胞生长、增殖、分化及迁移等过程,常氧下(体积分数21%O2)也有表达,但是低氧诱导因子1α中的402位和564位的脯氨酸残基被脯氨酰羟化酶羟化后与肿瘤抑制蛋白结合发生泛素化形成肿瘤抑制蛋白复合物,最终被蛋白酶水解,同时低氧诱导因子1α抑制因子抑制了低氧诱导因子1α的803位的天冬酰胺残基,阻止了803位点与CBP和p300的结合,从而降低了低氧诱导因子1α的活性。而在缺氧环境下α亚基不能发生羟基化,不能通过泛素化途径降解,细胞内低氧诱导因子1α蛋白水平升高,与低氧诱导因子1β形成异二聚体转移至细胞核内,结合缺氧反应元件HRE激活靶基因转录,调节机体适应低氧环境[14]。目前已有研究初步证实了低氧诱导因子1α在牙髓干细胞成骨/成牙本质分化中的促进/抑制作用,可能部分依赖于Wnt/β-catenin及Notch等信号通路[15-16]。除此之外,SHI等[17]通过生信分析识别和定位缺氧条件下牙髓干细胞的核心调节因子,结果提示STL基因参与了牙髓干细胞成骨/成牙本质分化能力的正向调控。研究报道低氧预处理可以改变牙髓干细胞小细胞囊泡的miRNA表达谱,富集并传递MiR-210-3p,靶向结合核转录因子κB下游基因,诱导巨噬细胞M2向极化以及抑制破骨细胞形成的能力均增强,可以促进组织修复及炎症性骨吸收的损伤,为骨修复再生的生物治疗提供了实验依据。随着细胞培养技术的不断发展,未来有必要进一步研究低氧状态下牙髓干细胞成骨/成牙本质分化的具体机制,为牙髓损伤下的修复再生提供理论依据及技术手段。 文章总结了低氧对牙髓干细胞成骨/成牙本质分化影响的研究进展[16-21],见表1。 "


2.2.2 力学刺激 细胞力学是组织工程学的一个重要组成部分,血液流动、肌肉收缩及心脏跳动等重要的生命活动都属于力学微环境的范畴。细胞外部的力学信号如剪切、拉伸、压缩和流体静压等均能够影响干细胞的增殖、自我更新和分化[22]。牙齿作为咀嚼器官,咀嚼过程中的机械力以及正畸过程中的矫治力等可通过牙釉质、牙本质传给髓腔内的牙髓组织,细胞处于三维动态的微环境中,成纤维细胞作为牙髓组织中的主要细胞类型,其未分化的状态为牙髓干细胞,牙髓干细胞受到颌骨运动及咬合力的机械作用,机械刺激通过受体激活细胞内的信号传导,探讨力学刺激对牙髓干细胞成骨/成牙本质分化的影响在正畸治疗以及骨缺损修复等方面具有重要意义,但是目前关于力学加载仪器以及加载时间顺序等方面未达成共识,关于力学刺激对牙髓干细胞成骨/成牙本质分化的研究较少,且结果存在不一致性。 机械刺激被细胞膜上的受体感知并转化为生物化学信号,这个过程是力学信号的传导过程,包括机械力在细胞骨架与蛋白分子链的传递及转导为生化因子信号激活转录子与转录调节子两条线。研究表明,细胞外基质是由氨基聚糖与蛋白聚糖等连续张力构件与胶原蛋白等不连续压缩构件等组成的张拉整体,不仅能为组织提供力学支持,其弹性和刚度还会影响细胞的增殖、分化及迁移等生物学特性。整合素是细胞膜上介导细胞与细胞外基质连接的力学感受蛋白,由α亚基(18种)和β亚基(8种)的不同组合而组成,主要包括胞外结构域、胞质结构域、跨膜结构域3个部分,细胞基于整合素的黏附力等与细胞外基质结合时,肌动蛋白收缩产生牵引力,通过不同程度的整合素和蛋白多糖聚集及相关离子通路及转录因子活性感测基质刚度的变化,改变细胞力学的传导而影响细胞的功能[23-24]。有学者研发了一种材料和培养系统来修改及测量细胞保留机械感累积效应的程度,揭示了机械力通过纤连蛋白的RGD黏附基序(细胞外基质的代表)对干细胞产生影响,为调控干细胞的力学感知提供了新途径[25]。 黏着斑是将细胞骨架与细胞外基质连接在一起的结构,可以将细胞外的力转导到胞内,Piezo蛋白作为一种离子通路,可以感知拉伸力、剪切力及渗透压等力学刺激,通过开放钙离子通道调节其下游通路,除此之外机械力还可以传递到细胞核,染色质结构变形后调节转录位点,启动基因的表达。接受力学刺激后细胞内部发生一系列变化,启动相关信号通路,如黏着斑激酶可以通过破坏整合素氨基端结构域和中央激酶结构域之间的自抑制分子内相互作用而激活,激活后与Src家族激酶形成复合体继续调节MAPK,Rho激酶和Wnt/β-catenin等下游信号通路[26]。关于力学刺激对牙髓干细胞的机制研究大多较为表浅且未能达成共识,MIYASHITA等[27]发现机械压应力可通过MAPK通路中的ERK1/2和p38信号通路诱导牙髓干细胞的成牙本质分化,同时骨形态发生蛋白7和Wnt10a 等特异性标记物的表达也上调。此外,HATA等[28]发现单轴机械拉伸应力可以通过PI3K/Akt和ERK等信号通路抑制牙髓干细胞的成骨分化能力。SHIBUTANI等[29]发现咬合刺激影响了牙髓内微血管的形态,从而刺激牙髓牙本质复合体内部的机械感受器引起牙髓内压力的变化,进而导致牙髓功能的减退。未来可以考虑在特定的细胞外基质中培养牙髓干细胞或者在特定的力学刺激下培养或者诱导牙髓干细胞的发育,为压力刺激所致牙髓损伤修复的应用提供新的思路。 文章总结了力学刺激对牙髓干细胞成骨/成牙本质分化影响的研究进展[27-28,30-34],见表2。 "


2.2.3 激光 自医疗激光治疗应用兴起以来,已被证明可以减轻疼痛、炎症、异常免疫反应以及促进组织愈合和再生,因其方向性好、操作简单和创伤小等特性在口腔临床中的应用也日益广泛,如牙齿修复、正畸、龋病、黏膜病以及牙周治疗等方面[35]。低强度激光治疗通过低能量、短脉宽、窄光谱的特定方式来调节生物活性,ARANY等[8]首次证实了低功率激光可激活体内干细胞再生组织的能力,利用低功率的激光处理牙髓组织,戴上临时冠后观察12周,高分辨率X射线成像和显微镜下观察到激光治疗下牙本质的形成增多,具体分子机制为激光以一种剂量依赖性的方式首先诱导了活性氧簇,通过特定的蛋氨酸残基激活潜伏的转化生长因子β1复合物,促使干细胞分化为牙本质。这一研究成果为之后的牙髓再生及骨修复方面奠定了科学基础。近几年来大多数研究表明低强度激光照射治疗对牙髓干细胞的增殖和牙源性分化起着积极作用,发光二极管作为一种新型光源,也可以通过电光转换产生类似激光作用的细胞生物效应进而影响干细胞的增殖分化,然而,还有一些研究呈现相反的结果,牙髓干细胞对不同类型激光照射的反应主要取决于照射的参数、所使用的波长、功率和能量密度等,激光治疗诱导各类型细胞增殖分化所需的参数设置仍待探究。 低强度激光治疗也称为光生物调节,是应用光子在非热作用下产生光化学效应,可以与植物的光合作用过程相比较,在植物的光合作用中,光子被细胞的光感受器吸收并引发化学变化。线粒体是细胞中的“化学工厂”,通过氧化磷酸化产生三磷酸腺苷,低强度激光能够光解离细胞色素C氧化酶中的一氧化氮,增加线粒体的膜电位,加速三磷酸腺苷的形成。低强度激光能够通过增加活性氧和减少活性氮使细胞整体氧化还原电位向更强的氧化方向转移,改变转录因子水平,激活多种信号传导通路,影响细胞的增殖、分化、凋亡等生物学特性[36]。发光二极管红光可以刺激电子传递链中细胞色素C氧化酶的铜/血红素铁中心,使活性氧和三磷酸腺苷的生成增多,有研究发现4 J/cm2的发光二极管红光促进炎症环境下牙髓干细胞成骨/成牙本质向分化,其作用机制可能为通过上调ERK1/2、JNK、ERK5等MAPK的亚信号通路减少炎症因子肿瘤坏死因子α和白细胞介素1β的释放[37]。TRPV1是一种Ca2+离子通道,JIAQI等[38]研究发现蓝色发光二极管照射可以增加TRPV1的活性和细胞内Ca2+水平,此外经选择性TRPV1抑制剂预处理后能够消除蓝色发光二极管的成骨分化作用,TRPV1/Ca2+可能是蓝光发光二极管诱导的成骨过程中重要的信号通路。激光治疗操作简单、成本低廉、临床转化障碍低,作为一种有前途的无毒治疗手段,为口腔颌面部组织再生中的应用提供了可能,但仍需补充相关研究确定其最佳照射剂量和功率输出。 文章总结了激光对牙髓干细胞成骨/成牙本质分化影响的研究进展[37-43],见表3。"

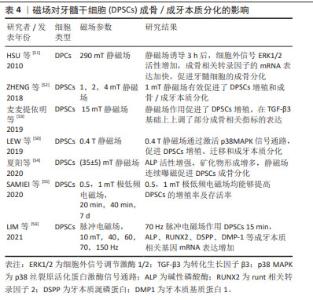
2.2.4 磁场 地球上的一切生物体一直暴露于各种类型的磁场环境中,磁场分为静磁场和动磁场,磁场作用于人体主要是通过磁力线穿透人体作用于细胞产生感应电流,其效应主要通过控制强度、频率和暴露时间来调节,影响各种细胞的迁移、增殖和分化,被广泛应用于相关疾病的物理疗法,如高血压、糖尿病等。当组织发生炎症或损伤时,炎症损伤相关部位会优先吸引移植入人体的外源性间充质干细胞归巢,进而发挥治疗作用[44]。于是,相关学者对此展开了大量研究,如基因改造、磁引导技术、水凝胶支架等,有效地克服了干细胞治疗中靶向率低的难点,其中磁引导技术即用磁性颗粒标记干细胞,之后利用磁场吸引至损伤部位,经低剂量磁性纳米粒子与静磁场预处理后间充质干细胞产生的外泌体,可以通过外泌体中miR-1260a靶向HDAC7和COL4A2增强成骨和血管生成能力[45],磁场与电场相互依存、相互联系形成电磁场,脉冲电磁场是一种高能非电离辐射,产生的感应电流直接作用于细胞膜,从而对细胞表面产生电化作用,脉冲电磁场可以刺激种植体在早期愈合中的稳定性[46]。许多研究表明磁场对牙髓干细胞的成骨/成牙本质向分化有促进作用,磁场技术为骨组织工程提供了一种新的技术方法,但是由于窗口效应,磁场强度、暴露时间等相关磁场参数范围仍需进一步观察。 磁场技术在生物医学领域极具应用潜力,磁场的作用机制可能与生物膜有关,目前的相关作用机制表明磁场可通过影响相关离子浓度及多种信号通路调控干细胞的生物学功能。磁现象的本质是电荷运动,人体体液是电解质溶液,属于导体,磁力线切割导体产生感应电流,在交变磁场作用下Na+、Cl-、K+等活动加强,改变膜电位,细胞膜的通透性增加,促进了细胞内外物质交换。WU 等[47]研究发现静磁场通过调控MAPK信号通路中的ERK和JNK蛋白磷酸化介导间充质干细胞的增殖,且T型钙离子通道是响应磁场信号的重要感应器。还有研究提出锌离子和2- APB敏感通路作为新的候选机制参与到磁场与干细胞的相互作用且锌离子也成为该领域的焦点[48]。静磁场可以通过影响牙髓干细胞的细胞膜激活胞内钙离子,激活p38 MAPK信号通路,重组细胞骨架,促进牙髓干细胞细胞增殖[49-50],还可以使细胞外信号ERK1/2活性增加,使成骨相关转录因子的mRNA表达加快,促进牙髓干细胞的成骨分化[51]。ZHENG等[52]研究表明转录共激活因子YAP/TAZ参与了牙髓干细胞的成骨/成牙本质向分化,同时肌动蛋白可能参与了YAP/TAZ的核定位。磁场可几乎无衰减地进入组织到达细胞特定部位,利用先进技术将磁场作用机制的研究引入干细胞领域,可以有效促进磁场在疾病治疗中的应用。 文章总结了磁场对牙髓干细胞成骨/成牙本质分化影响的研究进展[50-56],见表4。"


2.2.5 微重力 地球上存在的许多物理现象都与重力因素相关,生命体在太空环境中受到的重力水平为地球表面的百万分之一,在微重力环境下能观察到许多地球上不可能出现的物理现象。过去20年,再生医学以及空间站技术迅速发展,航空作业以及持续微重力环境可以影响干细胞的自我更新以及多向分化的能力,在疾病建模、生物制造以及干细胞衍生产品等方面均崭露头角[57]。近些年来,各项太空实验正有序开展,由于太空实验的局限性,许多学者通过在地面模拟微重力等方式以探讨微重力对干细胞增殖分化的影响。不同于地面培养,微重力环境为细胞提供了更接近体内的三维培养空间,使细胞更接近体内的环境,目前主要有两条技术路线,一是采用飞船、空间站等进行实际空间科学实验,二是通过物理仿真手段等进行地面模拟实验。LI等[58]在模拟微重力下诱导间充质干细胞成骨和成脂分化,通过RNA测序分析成骨分化过程中转录组的表达,结果发现大部分富集的差异表达基因与细胞周期和分裂相关,模拟微重力在成骨早期可抑制间充质干细胞的增殖,但在中期可促进其成骨分化和成脂分化。MAYER-WAGNER等[59]发现模拟微重力可抑制间充质干细胞的成骨潜能,但是与低频电磁场联合作用时可逆转被抑制的成骨潜能。牙髓干细胞作为一种有前途的间充质干细胞,探究微重力作用下牙髓干细胞生物学行为的改变,为牙组织工程提供了思路和线索。动态培养能够更加准确地模拟细胞在体内的生长环境及细胞间相互作用,许多结果表明微重力在牙髓干细胞的分化等方面起着重要作用但结果尚未统一,且尚有许多关键问题未得到解决,需要进行更多的研究以创建更适宜的微重力环境,推进微重力在临床上的应用。 关于模拟微重力下影响牙髓干细胞分化的机制研究方面目前尚处于探索阶段,有研究发现在微重力环境下,包括胶原家族成员、碱性磷酸酶及RUNT相关转录因子2等10个成骨特异性基因的表达减少,脂肪因子及瘦素等4个成脂特异性基因的表达增加,推测空间微重力可能通过骨形态发生蛋白2/SMAD信号通路和整合素/FAK/ERK通路降低 RUNT相关转录因子2的表达和活性,进而影响 G1期的细胞周期进展来抑制干细胞成骨向分化,p38 MAPK活性的增加和AKT活性的降低可能同步导致成脂相关信号通路的激活[60-61]。Rho A-Rho激酶信号通路已被证明参与多种以肌动蛋白为基础的生物学过程,如细胞骨架的重排等,牛玉梅等[62]进一步发现在模拟微重力下Rho A蛋白表达水平下调,Rho激酶的表达降低,成骨/成牙本质相关基因的mRNA表达减少,提示模拟微重力可能通过调控Rho A-Rho激酶信号通路抑制牙髓干细胞的矿化。另外也有研究表明,模拟微重力条件下干细胞的分化与能量代谢也存在潜在的关系,LIU等[63]研究表明模拟微重力下能量传感器Sirt1表达下调,抑制氧化磷酸化,抑制间充质干细胞的成骨分化;利用Sirt1的激活剂白藜芦醇上调Sirt1的表达后,氧化磷酸化及干细胞的成骨分化均得以恢复。微重力可以通过多种重要的信号通路来调控干细胞的分化,在太空微重力下进行干细胞的研究潜力巨大,能否利用微重力开展大规模的组织工程的构建也是当前的热点问题,有必要进一步阐明空间微重力下影响干细胞分化的具体作用机制,为牙髓干细胞分化提供新的实验模型,为再生医学的发展提供独特的技术路径。 文章总结了微重力对牙髓干细胞成骨/成牙本质分化影响的研究进展[62,64-67],见表5。"

| [1] KONG Y, DUAN J, LIU F, et al. Regulation of stem cell fate using nanostructure-mediated physical signals. Chem Soc Rev. 2021;50(22): 12828-12872. [2] GRONTHOS S, MANKANI M, BRAHIM J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625-13630. [3] MIURA M, GRONTHOS S, ZHAO M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10): 5807-5812. [4] GANDIA C, ARMINAN A, GARCIA-VERDUGO JM, et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2008;26(3):638-645. [5] GOMES JA, GERALDES MB, MELO GB, et al. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Invest Ophthalmol Vis Sci. 2010;51(3):1408-1414. [6] YAMAZA T, KENTARO A, CHEN C, et al. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther. 2010;1(1):5. [7] SAKAI K, YAMAMOTO A, MATSUBARA K, et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122(1):80-90. [8] ARANY PR, CHO A, HUNT TD, et al. Photoactivation of endogenous latent transforming growth factor-beta1 directs dental stem cell differentiation for regeneration. Sci Transl Med. 2014;6(238):238ra69. [9] XUAN K, LI B, GUO H, et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 2018;10(455):eaaf3227. [10] LI W, JIAO X, SONG J, et al. Therapeutic potential of stem cells from human exfoliated deciduous teeth infusion into patients with type 2 diabetes depends on basal lipid levels and islet function. Stem Cells Transl Med. 2021;10(7):956-967. [11] ZHANG XM, OUYANG YJ, YU BQ, et al. Therapeutic potential of dental pulp stem cell transplantation in a rat model of Alzheimer’s disease. Neural Regen Res. 2021;16(5):893-898. [12] HAN Y, KOOHI-MOGHADAM M, CHEN Q, et al. HIF-1alpha stabilization boosts pulp regeneration by modulating cell metabolism. J Dent Res. 2022;101(10):1214-1226. [13] YU CY, BOYD NM, CRINGLE SJ, et al. Oxygen distribution and consumption in rat lower incisor pulp. Arch Oral Biol. 2002;47(7):529-536. [14] KOYASU S, KOBAYASHI M, GOTO Y, et al. Regulatory mechanisms of hypoxia-inducible factor 1 activity: two decades of knowledge. Cancer Sci. 2018;109(3):560-571. [15] 关丽娜,杨帆,尹东锋,等.低氧环境下Notch信号通路对人牙髓干细胞成牙本质向分化的影响[J].中华口腔医学研究杂志(电子版),2020,14(4):214-220. [16] SHION O, NOBUYUKI K, KENTO T, et al. Hypoxia-inducible factor 1α induces osteo/odontoblast differentiation of human dental pulp stem cells via Wnt/β-catenin transcriptional cofactor BCL9. Scientific Reports. 2022;12(1):682. [17] SHI R, YANG H, LIN X, et al. Analysis of the characteristics and expression profiles of coding and noncoding RNAs of human dental pulp stem cells in hypoxic conditions. Stem Cell Res Ther. 2019; 10(1):89. [18] BELLAH ANE, MASASHI M, SATORU K, et al. The effects of hypoxia on the stemness properties of human dental pulp stem cells (DPSCs). Scientific reports. 2016;6(1):35476. [19] YAN W, FANG H, XIN Z, et al. Hypoxic preconditioning enhances dental pulp stem cell therapy for infection-caused bone destruction. Tissue engineering. Part A. 2016;22(19-20):1191-1203. [20] ANNA L, NATALIA B, ANDRZEJ K, et al. Multilineage differentiation potential of human dental pulp stem cells-impact of 3D and hypoxic environment on osteogenesis in vitro. Int J Mol Sci. 2020;21(17):6172. [21] RYAN P, YESSENIA V, RAGHUVARAN N, et al. C5a complement receptor modulates odontogenic dental pulp stem cell differentiation under hypoxia. Connect Tissue Res. 2021;63(4):339-348. [22] VINING KH, MOONEY DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol. 2017;18(12):728-742. [23] CHAUDHURI O, COOPER-WHITE J, JANMEY PA, et al. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature. 2020; 584(7822):535-546. [24] CHENG B, WAN W, HUANG G, et al. Nanoscale integrin cluster dynamics controls cellular mechanosensing via FAKY397 phosphorylation. Sci Adv. 2020;6(10):x1909. [25] XIE C, TANG H, LIU G, et al. Molecular mechanism of Epimedium in the treatment of vascular dementia based on network pharmacology and molecular docking. Front Aging Neurosci. 2022;14:940166. [26] HU D, DONG Z, LI B, et al. Mechanical force directs proliferation and differentiation of stem cells. Tissue Eng Part B Rev. 2023;29(2):141-150. [27] MIYASHITA S, AHMED NE, MURAKAMI M, et al. Mechanical forces induce odontoblastic differentiation of mesenchymal stem cells on three-dimensional biomimetic scaffolds. J Tissue Eng Regen Med. 2017;11(2):434-446. [28] HATA M, NARUSE K, OZAWA S, et al. Mechanical stretch increases the proliferation while inhibiting the osteogenic differentiation in dental pulp stem cells. Tissue Eng Part A. 2013;19(5-6):625-633. [29] SHIBUTANI N, HOSOMICHI J, ISHIDA Y, et al. Influence of occlusal stimuli on the microvasculature in rat dental pulp. Angle Orthod. 2010; 80(2):316-321. [30] YU V, DAMEK-POPRAWA M, NICOLL SB, et al. Dynamic hydrostatic pressure promotes differentiation of human dental pulp stem cells. Biochem Biophys Res Commun. 2009,386(4):661-665. [31] CAI X, ZHANG Y, YANG X, et al. Uniaxial cyclic tensile stretch inhibits osteogenic and odontogenic differentiation of human dental pulp stem cells. J Tissue Eng Regen Med. 2011;5(5):347-353. [32] 肖敏,陈博,李明伟,等.机械压应力刺激对人牙髓干细胞体外增殖矿化的影响[J].牙体牙髓牙周病学杂志,2015,25(4):187-192. [33] YANG H, SHU YX, WANG LY, et al. Effect of cyclic uniaxial compressive stress on human dental pulp cells in vitro. Connect Tissue Res. 2018; 59(3):255-262. [34] 李峻青,何文喜,郭倩,等.流体静水压力对牙髓干细胞成牙/成骨分化的影响[J].中国组织工程研究,2021,25(31):4976-4980. [35] FEKRAZAD R, ARANY P. Photobiomodulation therapy in clinical dentistry. Photobiomodul Photomed Laser Surg. 2019;37(12):737-738. [36] COTLER HB, CHOW RT, HAMBLIN MR, et al. The use of low level laser therapy (LLLT) for musculoskeletal pain. MOJ Orthop Rheumatol. 2015;2(5):00068. [37] 刘源,惠以宁,姜冰,等.LED红光上调MAPK信号促进炎性环境中人牙髓干细胞成骨/成牙本质分化[J].口腔疾病防治,2023,31(10): 701-711. [38] JIAQI C, YIMENG S, JIAYING L, et al. Low-level controllable blue LEDs irradiation enhances human dental pulp stem cells osteogenic differentiation via transient receptor potential vanilloid 1. J Photochem Photobiol B. 2022;233:112472. [39] ZACCARA IM, GINANI F, MOTA-FILHO HG, et al. Effect of low-level laser irradiation on proliferation and viability of human dental pulp stem cells. Lasers Med Sci. 2015;30(9):2259-2264. [40] ANNA T, ATHINA B, ELEANA K, et al. Odontogenic differentiation and biomineralization potential of dental pulp stem cells inside Mg-based bioceramic scaffolds under low-level laser treatment. Lasers Med Sci. 2017;32(1):201-210. [41] PINHEIRO C, DE PINHO MC, ARANHA AC, et al. Low power laser therapy: a strategy to promote the osteogenic differentiation of deciduous dental pulp stem cells from cleft lip and palate patients. Tissue Eng Part A. 2018;24(7-8):569-575. [42] BIDAR M, BAHLAKEH A, MAHMOUDI M, et al. Does the application of GaAlAs laser and platelet-rich plasma induce cell proliferation and increase alkaline phosphatase activity in human dental pulp stem cells? Lasers Med Sci. 2021;36(6):1289-1295. [43] AMID R, KADKHODAZADEH M, GILVARI SM, et al. Effects of two protocols of low-level laser therapy on the proliferation and differentiation of human dental pulp stem cells on sandblasted titanium discs: an in vitro study. J Lasers Med Sci. 2022;13:e1. [44] BELEMA-BEDADA F, UCHIDA S, MARTIRE A, et al. Efficient homing of multipotent adult mesenchymal stem cells depends on FROUNT-mediated clustering of CCR2. Cell Stem Cell. 2008;2(6):566-575. [45] WU D, CHANG X, TIAN J, et al. Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: release of exosomal miR-1260a improves osteogenesis and angiogenesis. J Nanobiotechnol. 2021;19(1):209. [46] NAYAK BP, DOLKART O, SATWALEKAR P, et al. Effect of the pulsed electromagnetic field (PEMF) on dental implants stability: a randomized controlled clinical trial. Materials (Basel). 2020;13(7): 1667. [47] WU H, LI C, MASOOD M, et al. Static magnetic fields regulate t-type calcium ion channels and mediate mesenchymal stem cells proliferation. Cells. 2022;11(15):2460. [48] OZGUN A, GARIPCAN B. Magnetic field-induced Ca(2+) intake by mesenchymal stem cells is mediated by intracellular Zn(2+) and accompanied by a Zn(2+) influx. Biochim Biophys Acta Mol Cell Res. 2021;1868(9):119062. [49] LEW WZ, HUANG YC, HUANG KY, et al. Static magnetic fields enhance dental pulp stem cell proliferation by activating the p38 mitogen-activated protein kinase pathway as its putative mechanism. J Tissue Eng Regen Med. 2018;12(1):19-29. [50] LEW WZ, FENG SW, LIN CT, et al. Use of 0.4-Tesla static magnetic field to promote reparative dentine formation of dental pulp stem cells through activation of p38 MAPK signalling pathway. Int Endod J. 2019;52(1):28-43. [51] HSU SH, CHANG JC. The static magnetic field accelerates the osteogenic differentiation and mineralization of dental pulp cells. Cytotechnology. 2010;62(2):143-155. [52] ZHENG L, ZHANG L, CHEN L, et al. Static magnetic field regulates proliferation, migration, differentiation, and YAP/TAZ activation of human dental pulp stem cells. J Tissue Eng Regen Med. 2018;12(10): 2029-2040. [53] 麦麦提依明·哈力克,热孜亚·艾尼,陈晓涛,等.恒定磁场作用下TGF-β3对兔牙髓干细胞成骨分化潜能的体外研究[J].临床口腔医学杂志,2019,35(12):719-723. [54] 夏阳,陈慧敏,胡姝颖,等.静磁场连续曝磁对牙髓干细胞增殖和分化的影响[J].南京医科大学学报(自然科学版),2020,40(2): 191-194. [55] SAMIEI M, AGHAZADEH Z, ABDOLAHINIA ED, et al. The effect of electromagnetic fields on survival and proliferation rate of dental pulp stem cells. Acta Odontol Scand. 2020;78(7):494-500. [56] HANMOI L, MYEONGHYUN N, YUMI K, et al. Increasing odontoblast-like differentiation from dental pulp stem cells through increase of β-catenin/p-GSK-3β expression by low-frequency electromagnetic field. Biomedicines. 2021;9(8):1049. [57] SHARMA A, CLEMENS RA, GARCIA O, et al. Biomanufacturing in low Earth orbit for regenerative medicine. Stem Cell Reports. 2022;17(1):1-13. [58] LI L, ZHANG C, CHEN JL, et al. Effects of simulated microgravity on the expression profiles of RNA during osteogenic differentiation of human bone marrow mesenchymal stem cells. Cell Prolif. 2019;52(2):e12539. [59] MAYER-WAGNER S, HAMMERSCHMID F, BLUM H, et al. Effects of single and combined low frequency electromagnetic fields and simulated microgravity on gene expression of human mesenchymal stem cells during chondrogenesis. Arch Med Sci. 2018;14(3):608-616. [60] CUI Z, LIANG L, YUANDA J, et al. Space microgravity drives transdifferentiation of human bone marrow-derived mesenchymal stem cells from osteogenesis to adipogenesis. FASEB J. 2018;32(8): 4444-4458. [61] 杨典凇,潘爽,何丽娜,等.整合素α6对模拟微重力下人牙髓干细胞粘附能力的影响[J].口腔医学研究,2016,32(4):361-364. [62] 牛玉梅,张巍巍,曹涛,等.模拟微重力影响人牙髓干细胞的矿化能力与RhoA-Rho激酶信号通路相关性研究[J].口腔医学,2016, 36(5):399-402. [63] LIU L, CHENG Y, WANG J, et al. Simulated microgravity suppresses osteogenic differentiation of mesenchymal stem cells by inhibiting oxidative phosphorylation. Int J Mol Sci. 2020;21(24):9747. [64] 张锋,邓旭亮,梅芳,等.空间微重力环境对人牙髓间充质细胞的影响初探[J].科技导报,2007,25(2):34-37. [65] 费晓磊,张巍巍,李艳萍,等.模拟微重力对人牙髓干细胞-PLGA复合物矿化的影响[J].口腔医学研究,2013,29(2):135-137. [66] HE L, PAN S, LI Y, et al. Increased proliferation and adhesion properties of human dental pulp stem cells in PLGA scaffolds via simulated microgravity. Int Endod J. 2016;49(2):161-173. [67] LI Y, HE L, PAN S, et al. Three-dimensional simulated microgravity culture improves the proliferation and odontogenic differentiation of dental pulp stem cell in PLGA scaffolds implanted in mice. Mol Med Rep. 2017;15(2):873-878. [68] TING G, CHIN HB, MAN LEC, et al. Current advance and future prospects of tissue engineering approach to dentin/pulp regenerative therapy. Stem Cells International. 2016;2016:9204574. [69] ZHOU M, LIU NX, SHI SR, et al. Effect of tetrahedral DNA nanostructures on proliferation and osteo/odontogenic differentiation of dental pulp stem cells via activation of the notch signaling pathway. Nanomedicine. 2018;14(4):1227-1236. [70] ALSHEMARY AZ, PAZARÇEVIREN AE, KESKIN D, et al. Porous clinoptilolite-nano biphasic calcium phosphate scaffolds loaded with human dental pulp stem cells for load bearing orthopedic applications. Biomed Mater. 2019;14(5):55010. [71] CHANG B, MA C, FENG J, et al. Dental pulp stem cell polarization: effects of biophysical factors. J Dent Res. 2021;100(10):1153-1160. [72] MOHAMMED E, BEHEREI HH, EL-ZAWAHRY M, et al. Osteogenic enhancement of modular ceramic nanocomposites impregnated with human dental pulp stem cells: an approach for bone repair and regenerative medicine. J Genet Eng Biotechnol. 2022;20(1):123. [73] AZARYAN E, HANAFI-BOJD MY, ALEMZADEH E, et al. Effect of PCL/nHAEA nanocomposite to osteo/odontogenic differentiation of dental pulp stem cells. BMC Oral Health. 2022;22(1):505. [74] EJEIAN F, BAHARVAND H, NASR-ESFAHANI M H. Hedgehog signalling is dispensable in the proliferation of stem cells from human exfoliated deciduous teeth. Cell Biol Int. 2014;38(4):480-487. [75] TIAN Y, XU Y, FU Q, et al. Osterix is required for Sonic hedgehog-induced osteoblastic MC3T3-E1 cell differentiation. Cell Biochem Biophys. 2012;64(3):169-176. [76] MA D, YU H, XU S, et al. Stathmin inhibits proliferation and differentiation of dental pulp stem cells via sonic hedgehog/Gli. J Cell Mol Med. 2018;22(7):3442-3451. [77] KORNSUTHISOPON C, CHANSAENROJ A, MANOKAWINCHOKE J, et al. Non-canonical Wnt signaling participates in Jagged1-induced osteo/odontogenic differentiation in human dental pulp stem cells. Sci Rep. 2022;12(1):7583. [78] ZHONG TY, ZHANG ZC, GAO YN, et al. Loss of Wnt4 expression inhibits the odontogenic potential of dental pulp stem cells through JNK signaling in pulpitis. Am J Transl Res. 2019;11(3):1819-1826. [79] ZENG K, KANG Q, LI Y, et al. EVL promotes osteo-/odontogenic differentiation of dental pulp stem cells via activating JNK signaling pathway. Stem Cells Int. 2023;2023:7585111. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [3] | Yin Lu, Jiang Chuanfeng, Chen Junjie, Yi Ming, Wang Zihe, Shi Houyin, Wang Guoyou, Shen Huarui. Effect of Complanatoside A on the apoptosis of articular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1541-1547. |
| [4] | Hu Taotao, Liu Bing, Chen Cheng, Yin Zongyin, Kan Daohong, Ni Jie, Ye Lingxiao, Zheng Xiangbing, Yan Min, Zou Yong. Human amniotic mesenchymal stem cells overexpressing neuregulin-1 promote skin wound healing in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1343-1349. |
| [5] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [6] | Li Dijun, Jiu Jingwei, Liu Haifeng, Yan Lei, Li Songyan, Wang Bin. Three-dimensional gelatin microspheres loaded human umbilical cord mesenchymal stem cells for chronic tendinopathy repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1356-1362. |
| [7] | Lou Guo, Zhang Min, Fu Changxi. Exercise preconditioning for eight weeks enhances therapeutic effect of adipose-derived stem cells in rats with myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1363-1370. |
| [8] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [9] | Huang Ting, Zheng Xiaohan, Zhong Yuanji, Wei Yanzhao, Wei Xufang, Cao Xudong, Feng Xiaoli, Zhao Zhenqiang. Effects of macrophage migration inhibitory factor on survival, proliferation, and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1380-1387. |
| [10] | Aikepaer · Aierken, Chen Xiaotao, Wufanbieke · Baheti. Osteogenesis-induced exosomes derived from human periodontal ligament stem cells promote osteogenic differentiation of human periodontal ligament stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1388-1394. |
| [11] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [12] | Zhang Haojun, Li Hongyi, Zhang Hui, Chen Haoran, Zhang Lizhong, Geng Jie, Hou Chuandong, Yu Qi, He Peifeng, Jia Jinpeng, Lu Xuechun. Identification and drug sensitivity analysis of key molecular markers in mesenchymal cell-derived osteosarcoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1448-1456. |
| [13] | Xie Liugang, Cui Shuke, Guo Nannan, Li Aoyu, Zhang Jingrui. Research hotspots and frontiers of stem cells for Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1475-1485. |
| [14] | Li Jialin, Zhang Yaodong, Lou Yanru, Yu Yang, Yang Rui. Molecular mechanisms underlying role of mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1512-1522. |
| [15] | Cao Yue, Ye Xinjian, Li Biyao, Zhang Yining, Feng Jianying. Effect of extracellular vesicles for diagnosis and therapy of oral squamous cell carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1523-1530. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||