Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (27): 4306-4311.doi: 10.12307/2024.547
Previous Articles Next Articles
Research hotspots and trends in the field of articular cartilage repair: a visualization analysis
Zhang Zhilong1, 2, Yang Shengping1, 2, Chen Tianxin2, 3, Zhu Yuqi1, 2
- 1Ophthalmology Hospital, China Academy of Chinese Medical Sciences, Beijing 100000, China; 2China Academy of Chinese Medical Sciences, Beijing 100000, China; 3Wangjing Hospital, China Academy of Chinese Medical Sciences, Beijing 100000, China
-
Received:2023-09-26Accepted:2023-11-10Online:2024-09-28Published:2024-01-26 -
Contact:Zhu Yuqi, Master, Chief physician, Ophthalmology Hospital, China Academy of Chinese Medical Sciences, Beijing 100000, China; China Academy of Chinese Medical Sciences, Beijing 100000, China -
About author:Zhang Zhilong, Ophthalmology Hospital, China Academy of Chinese Medical Sciences, Beijing 100000, China; China Academy of Chinese Medical Sciences, Beijing 100000, China -
Supported by:the Second Special Project for International Cooperation in Traditional Chinese Medicine (Center-based Project) of the National Administration of Traditional Chinese Medicine, No. 0610-2040NF020931 (to ZYQ); Science and Technology Innovation Project of China Academy of Traditional Chinese Medicine, No. C12021A020 (to ZYQ)
CLC Number:
Cite this article
Zhang Zhilong, Yang Shengping, Chen Tianxin, Zhu Yuqi. Research hotspots and trends in the field of articular cartilage repair: a visualization analysis[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(27): 4306-4311.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
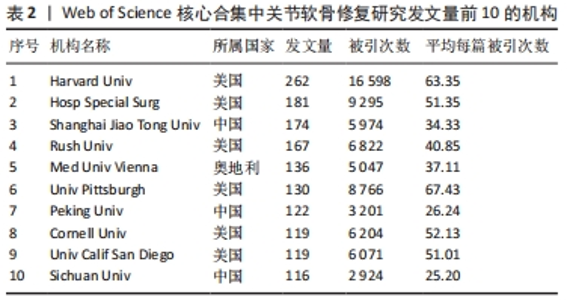
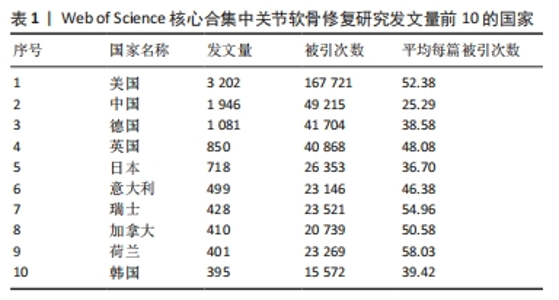
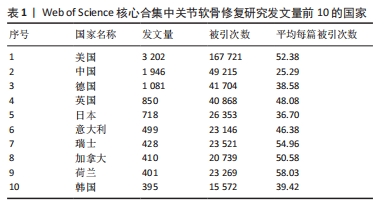
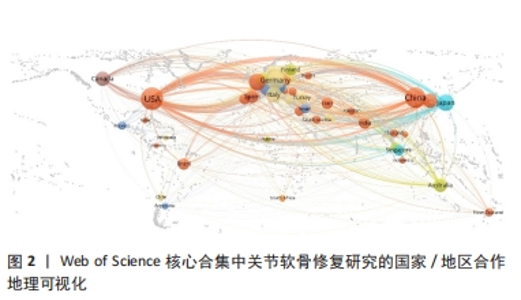
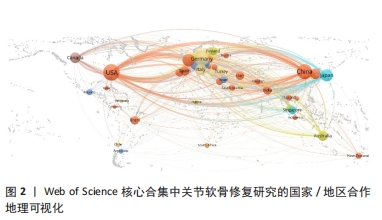
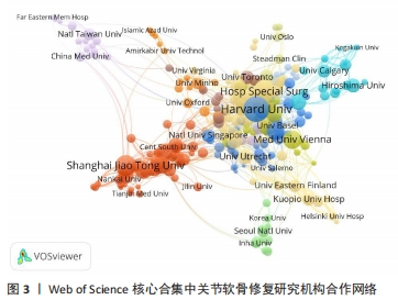
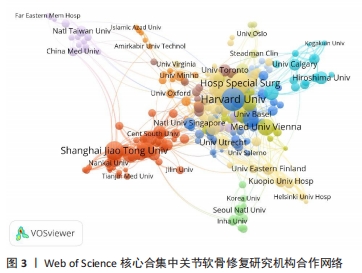
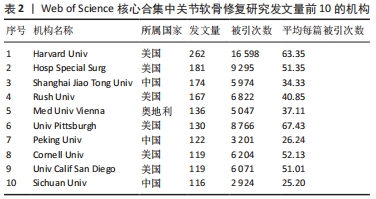
2.3 关节软骨修复领域研究机构分布 发文量作为衡量科研实力的一项重要指标,是一个科研机构实力强弱的重要表现。以已发表的文献作为参考项目,截至2023年8月共有6 639个机构参与了关节软骨修复的相关研究,哈佛大学、纽约特种外科医院、上海交通大学、拉什大学、维也纳医科大学、匹兹堡大学、北京大学、康奈尔大学、加州大学圣地亚哥分校、四川大学是发文量前10的研究机构,见表2。10所研究机构的发文量均在100篇以上,被引次数均超过3 000次,哈佛大学是该领域发文量和被引次数最多的研究机构,表明哈佛大学在该研究领域处于引领地位,匹兹堡大学是平均每篇被引次数最多的机构,表明其研究成果被众多学者所认同。由研究机构合作网络(图3)可知,多个研究机构之间建立了一定的合作关系,但这类合作有待进一步加强,尤其是增加不同机构间的跨国合作,可能更有利于该领域的发展。"
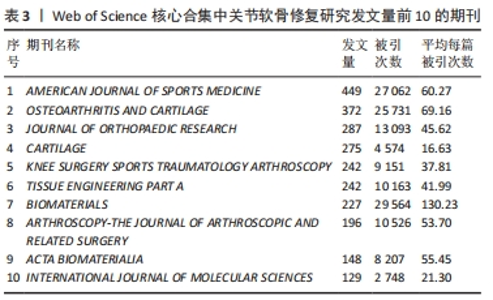
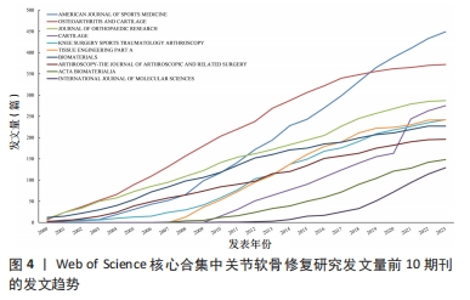
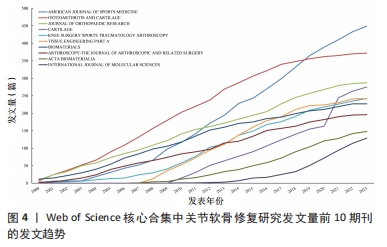
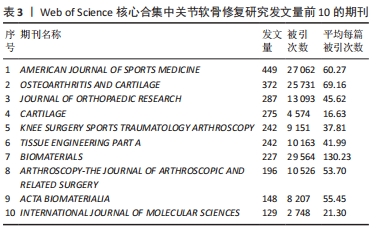
2.4 关节软骨修复领域研究期刊分布 对关节软骨修复领域研究的发表期刊进行统计分析,该领域出版文献最多的10个期刊的发文量均在100篇以上,文献被引次数均在2 000 次以上,平均每篇被引次数均大于16,见表3。《AMERICAN JOURNAL OF SPORTS MEDICINE(美国运动医学杂志)》是出版关节软骨修复相关文献最多的期刊,达到449篇,其次为《OSTEOARTHRITIS AND CARTILAGE(骨关节炎和软骨)》,为372篇。《BIOMATERIALS(生物材料)》杂志是该领域被引次数和平均每篇被引次数最多的期刊,表明其在该研究领域具有较高影响力,此期刊的文章一定程度上代表了该领域近年来的研究热点,表明生物材料研究在关节软骨修复中占有重要地位,可能是该领域的未来研究方向。"
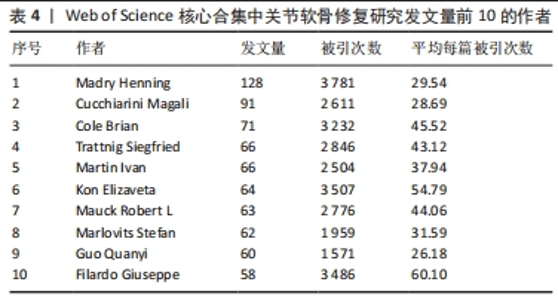
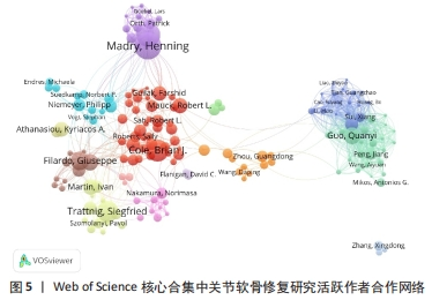
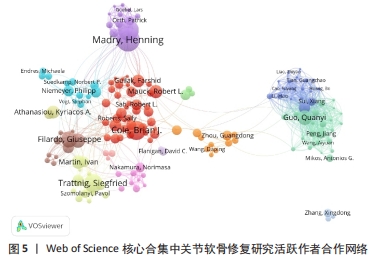
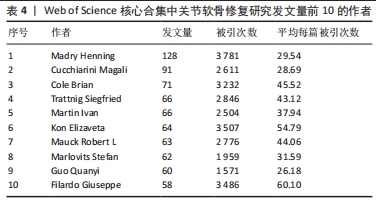
2.5 关节软骨修复领域研究作者合作分析 依据普莱斯定律计算核心作者发文量,计算公式为N=0.749×(ηmax)1/2(ηmax表示发文量最多作者的文献量),当作者发文量> N篇即为核心作者,经计算得出N=8.47,故该领域核心作者最低发文量为9篇,经VOSviewer 统计得出,自2000年以来该领域共有核心作者586位,共计发文8 898篇,占该领域总发文量的90.6%,说明关节软骨修复研究领域的核心作者群体贡献了绝大部分的研究成果。表4展示了发文量最多的10位作者,其中Madry Henning是该领域2000年1月至2023年8月期间发文最多(128篇)、被引次数最多(3 781次)的作者,表明其在该领域做出了相当大的贡献;Filardo Giuseppe是这一时期平均每篇被引次数最高的作者,达到60.10次。图5展示了发文量前200位活跃作者的合作网络。由图可知200位活跃作者相互之间形成了多个结构较为稳定的研究团队,各团队内部人员合作频繁,但不同团队间的合作相对缺乏,且有团队未与其他团队建立联系,表明不同团队间合作还有较大的增长空间。"
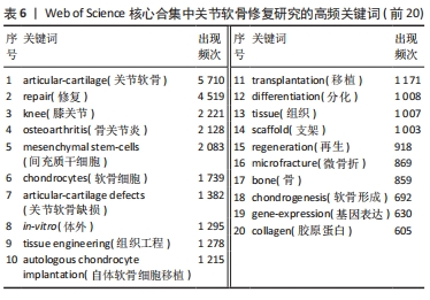
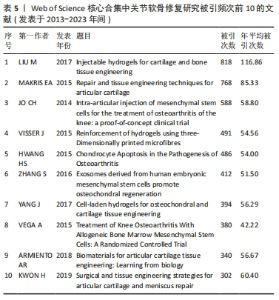
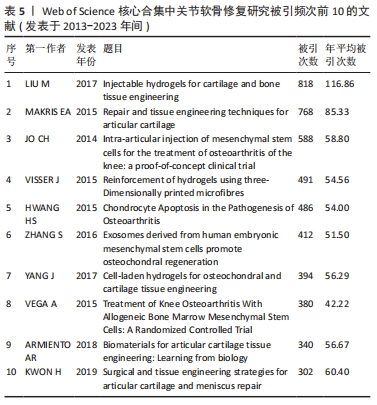
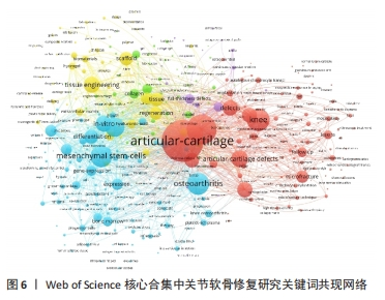
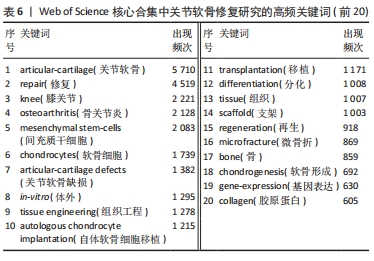
LIU 等[16]对软骨组织工程新型可注射水凝胶的生物材料选择和制备方法进行综述,认为可注射水凝胶是软骨组织工程很有前景的支架材料。MAKRIS等[17]对目前临床广泛应用的关节软骨表面缺损修复技术进行综述,认为组织工程在将生物技术转化为临床产品方面发挥了突出作用。JO等[18]发现关节腔内注射自体脂肪间充质干细胞可通过促进关节软骨再生和修复以治疗膝骨关节炎。VISSER等[19]发现凝胶/支架复合材料的刚度和弹性接近关节软骨组织,3D打印微纤维增强水凝胶的方法为生产具有生物和机械相容性的组织结构提供了基础。HWANG等[20]发现关节软骨损伤程度与软骨细胞凋亡之间存在相关性,认为软骨细胞凋亡或将成为调节关节软骨退变的有效靶点。ZHANG等[21]发现关节腔内注射人类胚胎间充质干细胞来源外泌体能有效修复关节软骨缺损。YANG等[22]回顾了促进骨软骨/软骨组织重建的细胞-水凝胶结构设计的最新进展,认为水凝胶/无机颗粒/干细胞复合材料在骨软骨/软骨组织工程中具有更好的物理和生物性能。VEGA等[23]发现关节腔内注射异体间充质干细胞治疗膝骨关节炎具有良好的可行性和安全性,能显著缓解膝关节疼痛和改善软骨质量。ARMIENTO等[4]介绍了软骨修复的现有治疗方法,并且回顾了软骨组织工程中不同生物材料的研究现状,认为组织工程和生物材料科学为关节软骨修复和再生带来了彻底的变革,目前相关临床转化尚未完全成功,但不断发展的新一代技术有望取得良好的结果。KWON等[24]认为组织工程方法正在成为软骨和半月板修复手术的替代方法,一些基于细胞和组织工程的产品正在进行临床试验,为软骨和半月板修复开辟了新的途径。 2.7 关节软骨修复领域研究关键词分析 关键词通常是文献内容的高度凝练总结,非常适合用于探索研究热点和趋势[25]。对关键词共现、聚类和突现情况进行分析可以更加全面地了解该领域研究的核心内容、热点和前沿,从而分析未来研究趋势,把握研究方向。 2.7.1 关键词共现 使用VOSviewer中的thesaurus terms.txt对关键词进行同义词合并后共得到10 891个关键词,对关键词出现频次进行统计,并利用 VOSviewer 构建关键词共现网络。由出现频次前20的高频关键词(表6)和关键词共现网络(图6)可知:骨关节炎、关节软骨缺损为该研究领域的热点疾病;膝关节为该领域研究最多的关节;干细胞疗法、组织工程、自体软骨细胞植入、微骨折、支架、胶原蛋白是备受重视的治疗方法及治疗物质;体外实验是重要的研究方法;软骨形成和再生、细胞分化及相关基因表达备受关注。"
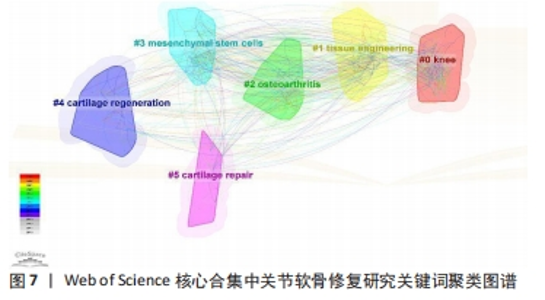
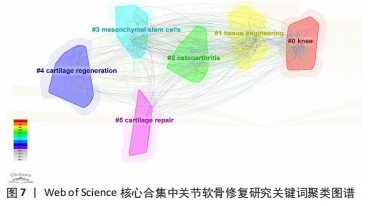
2.7.2 关键词聚类 运用Citespeace软件以对数似然比(LLR)方法进行关键词聚类分析,得到关键词聚类图,见图7,聚类模块值Q=0.442 3,平均轮廓值S=0.743 9,满足Q > 0.3,S > 0.5提示关键词聚类结构显著,且聚类结果同质性较高。共形成了#0 knee(膝关节)、#1 tissue engineering(组织工程)、#2 osteoarthritis(骨关节炎)、#3 mesenchymal stem-cells(间充质干细胞)、#4 cartilage regeneration(软骨再生)、#5 cartilage repair(软骨修复)6个有意义的聚类。各聚类间形成较为密集的连线,表明各聚类间联系紧密。其中,聚类#0代表主要研究的关节,可见研究人员对膝关节的关节软骨修复尤为重视;聚类#1、#3、#4、#5主要探讨关节软骨修复的具体干预措施和促进机制;聚类#2为相关疾病,表明关节软骨修复对骨关节炎的预防和治疗意义重大。"
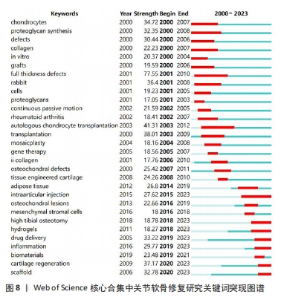
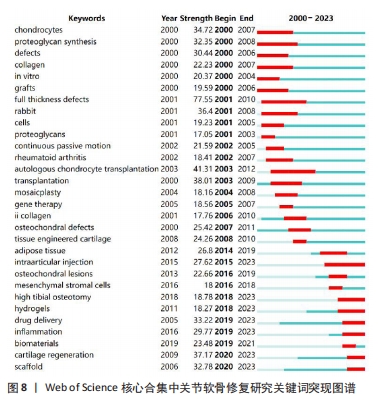
2.7.3 关键词突现 通过关键词突现分析可以找出一段时间内剧增的关键词,从更深度的层面观察该领域的发展变化[26]。关键词突现结果显示,full thickness defects(全层缺损)是关节软骨修复研究中突现强度最大的关键词,且与autologous chondrocyte transplantation(自体软骨细胞移植)均是突现时间最长的关键词,见图8。目前,仍处于突现状态的关键词有intraarticular injection(关节内注射)、high tibial osteotomy(胫骨高位截骨)、hydrogels(水凝胶)、drug delivery(药物递送)、inflammation(炎症)、cartilage regeneration(软骨再生)、scaffold(支架)。"
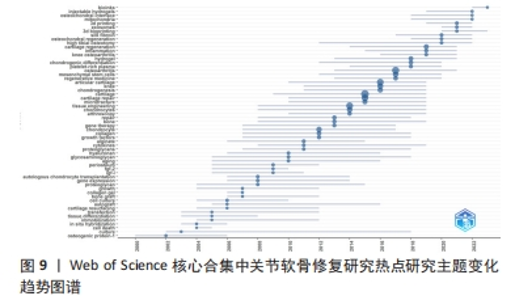
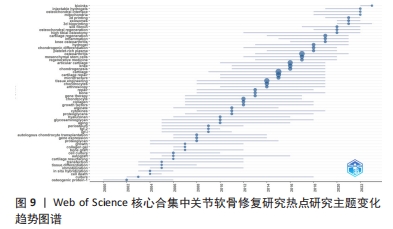
2.8 关节软骨修复领域热点研究主题变化趋势 运用Bibliometrix R-package 分析关节软骨修复领域热点研究主题的时间分布情况,见图9。自2000年以来,该研究领域共形成59个热点研究主题,近5年备受关注的研究主题包括platelet-rich plasma(富血小板血浆)、chondrogenic differentiation(软骨细胞分化)、hydrogel(水凝胶)、knee osteoarthritis(膝骨关节炎)、inflammation(炎症)、cartilage regeneration(软骨再生)、high tibial osteotomy(胫骨高位截骨术)、osteochondral regeneration(骨软骨再生)、silk fibroin(蚕丝蛋白)、3d bioprinting(3D生物打印)、exosomes(外泌体)、3d printing(3D打印技术)、mitochondria(线粒体)、osteochondral interface(骨软骨界面)、injectable hydrogels(可注射水凝胶)、bioinks[(用于3D打印的)生物墨水]。"
| [1] CARBALLO CB, NAKAGAWA Y, SEKIYA I, et al. Basic Science of Articular Cartilage. Clin Sports Med. 2017;36(3):413-425. [2] LUO Y, SINKEVICIUTE D, HE Y, et al. The minor collagens in articular cartilage. Protein Cell. 2017;8(8):560-572. [3] HUBER M, TRATTNIG S, LINTNER F. Anatomy, biochemistry, and physiology of articular cartilage. Invest Radiol. 2000;35(10):573-580. [4] ARMIENTO AR, STODDART MJ, ALINI M, et al. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018;65:1-20. [5] AHMED TA, HINCKE MT. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev. 2010;16(3):305-329. [6] GUERMAZI A, NIU J, HAYASHI D, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study). BMJ. 2012;345:e5339. [7] DING C, CICUTTINI F, JONES G. Tibial subchondral bone size and knee cartilage defects: relevance to knee osteoarthritis. Osteoarthritis Cartilage. 2007;15(5):479-486. [8] EVERHART JS, ABOULJOUD MM, FLANIGAN DC. Role of full-thickness cartilage defects in knee osteoarthritis (OA) incidence and progression: Data from the OA Initiative. J Orthop Res. 2019;37(1):77-83. [9] BORRELLI J JR, OLSON SA, GODBOUT C, et al. Understanding Articular Cartilage Injury and Potential Treatments. J Orthop Trauma. 2019;33(6):S6-S12. [10] MAKRIS EA, GOMOLL AH, MALIZOS KN, et al. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11(1):21-34. [11] CHENG K, ZHOU Y, WU H. Bibliometric analysis of global research trends on monkeypox: Are we ready to face this challenge? J Med Virol. 2023;95(1):e27892. [12] AHMAD P, SLOTS J. A bibliometric analysis of periodontology. Periodontol 2000. 2021; 85(1):237-240. [13] ARIA M, CUCCURULLO C. Bibliometrix: an R-tool for comprehensive science mapping analysis. J Informetrics. 2022;11(4):959-975. [14] TAN Y, SONG Q. Research trends and hotspots on the links between caveolin and cancer: bibliometric and visual analysis from 2003 to 2022. Front Pharmacol. 2023;14:1237456. [15] 孙宇康,宋丽娟,温春丽,等.基于Web of Science近十年干细胞治疗心肌梗死的可视化分析[J].中国组织工程研究,2024,28(7):1143-1148. [16] LIU M, ZENG X, Ma C, et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5:17014. [17] MAKRIS EA, GOMOLL AH, MALIZOS KN, et al. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11(1):21-34. [18] JO CH, LEE YG, SHIN WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254-1266. [19] VISSER J, MELCHELS FP, JEON JE, et al. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat Commun. 2015;6:6933. [20] HWANG HS, KIM HA. Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int J Mol Sci. 2015;16(11):26035-26054. [21] ZHANG S, CHU WC, LAI RC, et al. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24(12):2135-2140. [22] YANG J, ZHANG YS, YUE K, et al. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1-25. [23] VEGA A, MARTÍN-FERRERO MA, DEL CANTO F, et al. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation. 2015;99(8):1681-1690. [24] KWON H, BROWN WE, LEE CA, et al. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat Rev Rheumatol. 2019;15(9):550-570. [25] WANG S, ZHANG L, JIN Z, et al. Visualizing temporal dynamics and research trends of macrophage-related diabetes studies between 2000 and 2022: a bibliometric analysis. Front Immunol. 2023;14:1194738. [26] 洪靖,卢从飞,黄晨宾,等.材料生物力学研究热点与态势的可视化分析[J].中国组织工程研究,2024,28(15):2358-2363. [27] XUE S, RUAN G, LI J, et al. Bio-responsive and multi-modality imaging nanomedicine for osteoarthritis theranostics. Biomater Sci. 2023;11(15):5095-5107. [28] LIU W, MADRY H, CUCCHIARINI M. Application of Alginate Hydrogels for Next-Generation Articular Cartilage Regeneration. Int J Mol Sci. 2022;23(3):1147. [29] STACHEL N, ORTH P, ZURAKOWSKI D, et al. Subchondral Drilling Independent of Drill Hole Number Improves Articular Cartilage Repair and Reduces Subchondral Bone Alterations Compared With Debridement in Adult Sheep. Am J Sports Med. 2022; 50(10):2669-2679. [30] SHAMMA RN, SAYED RH, MADRY H, et al. Triblock Copolymer Bioinks in Hydrogel Three-Dimensional Printing for Regenerative Medicine: A Focus on Pluronic F127. Tissue Eng Part B Rev. 2022;28(2):451-463. [31] XU J, FAHMY-GARCIA S, WESDORP MA, et al. Effectiveness of BMP-2 and PDGF-BB Adsorption onto a Collagen/Collagen-Magnesium-Hydroxyapatite Scaffold in Weight-Bearing and Non-Weight-Bearing Osteochondral Defect Bone Repair: In Vitro, Ex Vivo and In Vivo Evaluation. J Funct Biomater. 2023;14(2):111. [32] VANNINI F, BERVEGLIERI L, BOFFA A, et al. Hyaluronic scaffold transplantation with bone marrow concentrate for the treatment of osteochondral lesions of the talus: durable results up to a minimum of 10 years. Knee Surg Sports Traumatol Arthrosc. 2023;31(10):4551-4558. [33] DI MARTINO A, PERDISA F, FILARDO G, et al. Cell-Free Biomimetic Osteochondral Scaffold for the Treatment of Knee Lesions: Clinical and Imaging Results at 10-Year Follow-up. Am J Sports Med. 2021;49(10):2645-2650. [34] ZAFFAGNINI S, ROMANDINI I, FILARDO G, et al. Meniscal allograft transplantation, anterior cruciate ligament reconstruction, and valgus high tibial osteotomy for meniscal-deficient, unstable, and varus knees: surgical technique and clinical outcomes. Int Orthop. 2023;47(10):2523-2535. [35] DI MARTINO A, SILVA S, ANDRIOLO L, et al. Osteochondral autograft transplantation versus autologous bone-cartilage paste grafting for the treatment of knee osteochondritis dissecans. Int Orthop. 2021;45(2):453-461. [36] GONG J, FAIRLEY J, CICUTTINI FM, et al. Effect of Stem Cell Injections on Osteoarthritis-related Structural Outcomes: A Systematic Review. J Rheumatol. 2021;48(4):585-597. [37] LEE SS, OH J, LEE DH. Change in Cartilage Status of Medial Compartment after Open-Wedge High Tibial Osteotomy without Cartilage Regeneration Procedure: Second Look Arthroscopic Assessment. Biomedicines. 2023;11(6):1639. [38] HAMAHASHI K, TOYODA E, ISHIHARA M, et al. Polydactyly-derived allogeneic chondrocyte cell-sheet transplantation with high tibial osteotomy as regenerative therapy for knee osteoarthritis. NPJ Regen Med. 2022;7(1):71. [39] WANG W, SHI Y, LIN G, et al. Advances in Mechanical Properties of Hydrogels for Cartilage Tissue Defect Repair. Macromol Biosci. 2023;23(7):e2200539. [40] QIU F, FAN X, CHEN W, et al. Recent Progress in Hydrogel-Based Synthetic Cartilage: Focus on Lubrication and Load-Bearing Capacities. Gels. 2023;9(2):144. [41] JONES IA, TOGASHI R, WILSON ML, et al. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol. 2019;15(2):77-90. [42] LI X, DAI B, GUO J, et al. Nanoparticle-Cartilage Interaction: Pathology-Based Intra-articular Drug Delivery for Osteoarthritis Therapy. Nanomicro Lett. 2021;13(1):149. [43] LOLLI A, SIVASUBRAMANIYAN K, VAINIERI ML, et al. Hydrogel-based delivery of antimiR-221 enhances cartilage regeneration by endogenous cells. J Control Release. 2019;309:220-230. [44] HE Y, SUN M, WANG J, et al. Chondroitin sulfate microspheres anchored with drug-loaded liposomes play a dual antioxidant role in the treatment of osteoarthritis. Acta Biomater. 2022;151:512-527. [45] LI M, YIN H, YAN Z, et al. The immune microenvironment in cartilage injury and repair. Acta Biomater. 2022;140:23-42. [46] TUAN RS, CHEN AF, KLATT BA. Cartilage regeneration. J Am Acad Orthop Surg. 2013; 21(5):303-311. [47] RAMZAN F, SALIM A, KHAN I. Osteochondral Tissue Engineering Dilemma: Scaffolding Trends in Regenerative Medicine. Stem Cell Rev Rep. 2023;19(6):1615-1634. [48] 张兴宇,陈世益.关节软骨修复和再生:从细胞到仿生支架药物递送研究进展[J].中国运动医学杂志,2021,40(1):67-72. |
| [1] | Yang Junliang, Lu Tan, Xu Biao, Jiang Yaqiong, Wang Fucheng. Three-dimensional finite element analysis of effects of partial anterior cruciate ligament rupture on knee joint stress [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1347-1353. |
| [2] | Yang Yufang, Yang Zhishan, Duan Mianmian, Liu Yiheng, Tang Zhenglong, Wang Yu. Application and prospects of erythropoietin in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1443-1449. |
| [3] | Chen Kaijia, Liu Jingyun, Cao Ning, Sun Jianbo, Zhou Yan, Mei Jianguo, Ren Qiang. Application and prospect of tissue engineering in treatment of osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1450-1456. |
| [4] | Mei Jingyi, Liu Jiang, Xiao Cong, Liu Peng, Zhou Haohao, Lin Zhanyi. Proliferation and metabolic patterns of smooth muscle cells during construction of tissue-engineered blood vessels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1043-1049. |
| [5] | Wang Shanshan, Shu Qing, Tian Jun. Physical factors promote osteogenic differentiation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1083-1090. |
| [6] | Xu Canli, He Wenxing, Wang Lei, Wu Fangting, Wang Jiahui, Duan Xuelin, Zhao Tiejian, Zhao Bin, Zheng Yang. Bibliometric analysis of researches on liver organoids [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1099-1104. |
| [7] | Sun Yukang, Song Lijuan, Wen Chunli, Ding Zhibin, Tian Hao, Ma Dong, Ma Cungen, Zhai Xiaoyan. Visualization analysis of stem cell therapy for myocardial infarction based on Web of Science in recent ten years [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1143-1148. |
| [8] | Zhang Kefan, Shi Hui. Research status and application prospect of cytokine therapy for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 961-967. |
| [9] | Chen Xiaofang, Zheng Guoshuang, Li Maoyuan, Yu Weiting. Preparation and application of injectable sodium alginate hydrogels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 789-794. |
| [10] | Wang Jiani, Chen Junyu. Angiogenesis mechanism of metal ions and their application in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 804-812. |
| [11] | Shen Ziqing, Xia Tian, Shan Yibo, Zhu Ruijun, Wan Haoxin, Ding Hao, Pan Shu, Zhao Jun. Vascularized tracheal substitutes constructed by exosome-load hydrogel-modified 3D printed scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 697-705. |
| [12] | Wang Jianchun, Yang Shuqing, Su Xin, Wang Hongyuan. Different contents of B2O3 affect mechanical properties and bioactivity of bioactive glass scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 712-716. |
| [13] | Zhu Liwei, Wang Jiangyue, Bai Ding. Application value of nanocomposite gelatin methacryloyl hydrogels in different bone defect environments [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 753-758. |
| [14] | Yang Yuqing, Chen Zhiyu. Role and application of early transient presence of M1 macrophages in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 594-601. |
| [15] | Yan Jie, Zhou Jing, Zhao Jingpu, Zhang Qingfang, Zhou Mingchao, Wang Yulong. Visual analysis of high-definition transcranial direct current stimulation research [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(32): 5110-5115. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||