Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (28): 4461-4468.doi: 10.12307/2024.491
Previous Articles Next Articles
Icariin regulates acidic microenvironment to alleviate pain caused by postmenopausal osteoporosis in the elderly
Xue Chunyang, Wang Xiuhui
- Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China
-
Received:2023-07-31Accepted:2023-09-02Online:2024-10-08Published:2023-11-27 -
Contact:Wang Xiuhui, Master, Chief physician, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China -
About author:Xue Chunyang, Master, Physician, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China -
Supported by:Clinical Characteristics Discipline Project of Shanghai Pudong New Area Health Commission, No. PWYts2021-03 (to WXH)
CLC Number:
Cite this article
Xue Chunyang, Wang Xiuhui. Icariin regulates acidic microenvironment to alleviate pain caused by postmenopausal osteoporosis in the elderly[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(28): 4461-4468.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
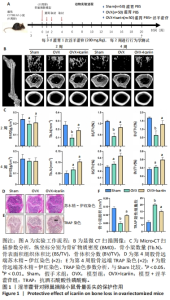
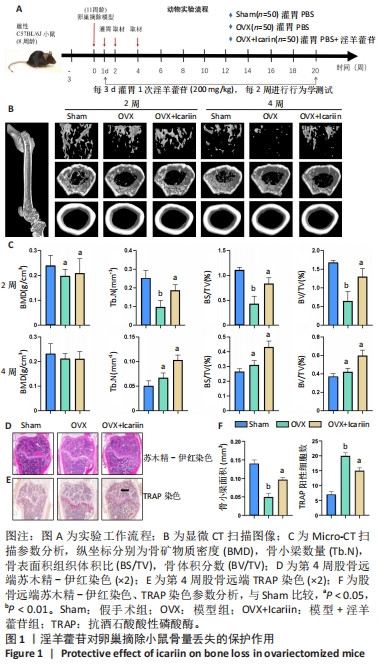
2.1 实验动物数量分析 实验共选择C57BL/6J小鼠200只,全部进入结果分析。 2.2 淫羊藿苷对卵巢摘除小鼠模型骨量损失有保护作用 2.2.1 动物实验 实验过程见图1A。3D重建图像及骨结构参数结果显示,与假手术组相比,模型组骨量损失显著增加,模型+淫羊藿苷组骨量损失通过淫羊藿苷治疗有效减轻,见图1B,C。股骨骨组织切片苏木精-伊红染色证实淫羊藿苷对卵巢摘除诱导的骨量丢失具有保护作用,见图1D。 2.2.2 细胞实验 淫羊藿苷对破骨细胞数量和功能的影响见图1E,F。骨切片TRAP活性染色显示,与模型组相比,模型+淫羊藿苷组骨小鼠骨组织中破骨细胞数量和破骨细胞在骨表面占据的表面积明显降低。"
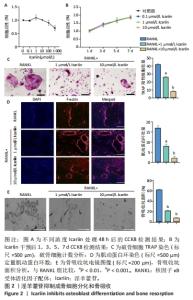
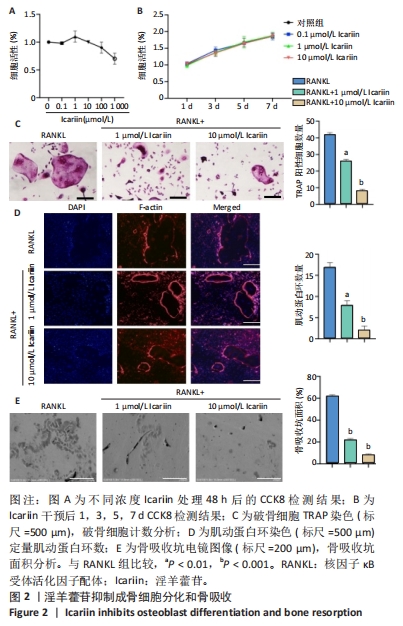
2.3 淫羊藿苷抑制成骨细胞分化和骨吸收 2.3.1 淫羊藿苷对骨髓巨噬细胞增殖活性的影响 随着淫羊藿苷浓度的增加,骨髓巨噬细胞的增殖活性降低,这表明淫羊藿苷对骨髓巨噬细胞增殖有浓度依赖性的抑制作用。值得注意的是,淫羊藿苷在1-10 μmol/L的浓度范围内,其增殖活性水平与对照组相似,表明相对缺乏毒性,见图2A;此外,在第1,3,5和7天进行测量时,获得了一致的结果,见图2B。 2.3.2 淫羊藿苷对破骨细胞分化的影响 RANKL和M-CSF分别在1 μmol/L和10 μmol/L淫羊藿苷浓度下诱导骨髓巨噬细胞分化,见图2C。与对照组相比,淫羊藿苷干预组TRAP阳性细胞明显减少;此外,淫羊藿苷10 μmol/L组的TRAP阳性细胞明显少于淫羊藿苷1 μmol/L组。结果表明淫羊藿苷具有剂量依赖性,可抑制骨髓巨噬细胞向破骨细胞的分化。 2.3.3 淫羊藿苷对肌动蛋白环形成及破骨细胞功能的影响 肌动蛋白环是RANKL诱导的成熟破骨细胞形成过程中的一个明显特征,见图2D。当骨髓巨噬细胞与RANKL共培养时,骨髓巨噬细胞分化为成熟的破骨细胞并形成肌动蛋白环。然而,随着淫羊藿苷浓度的增加,肌动蛋白环的大小和数量明显减少,表明淫羊藿苷抑制成熟破骨细胞肌动蛋白环的形成。 2.3.4 淫羊藿苷对骨吸收坑形成的影响 扫描电镜观察可见破骨细胞在骨片上形成较多的吸收坑,见图2E。然而,在淫羊藿苷的存在下,破骨细胞形成的吸收坑减少;其中RANKL+1 μmol/L淫羊藿苷和RANKL+10 μmol/L淫羊藿苷显著抑制破骨细胞骨吸收坑的形成。"
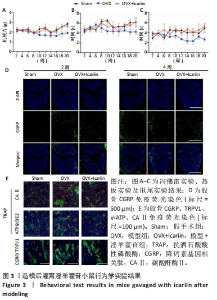
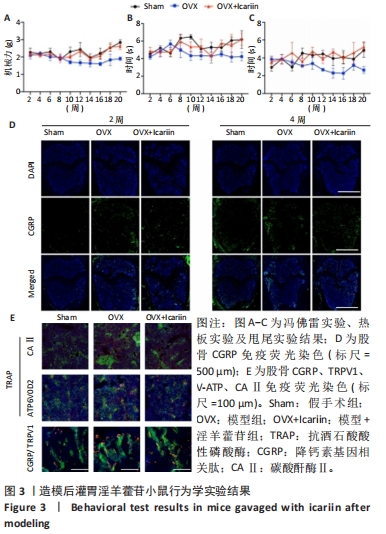
2.4 淫羊藿苷抑制卵巢摘除小鼠痛阈 2.4.1 淫羊藿苷对小鼠痛阈的影响 采用冯佛雷(Von Frey)试验评估机械异常性痛,热板实验和甩尾实验评估热痛觉过敏,见图3A-C。与假手术组相比,模型组小鼠的疼痛阈值明显升高,表明卵巢摘除导致小鼠疼痛阈值升高;与模型组相比,模型+淫羊藿苷组表现出更高的机械痛和热痛阈值。这些发现提示淫羊藿苷可能减轻机械异常性疼痛和热痛觉过敏,从而提高模型组小鼠的痛阈。 2.4.2 淫羊藿苷调节痛觉相关离子通道表达中的作用 已有研究报道,CGRP作为疼痛激活介质,在背根神经节激活后产生,参与外周和中枢神经源性疼痛信号的传递。随着淫羊藿苷干预时间的延长,与模型组相比,模型+淫羊藿苷组CGRP表达降低,见图3D。 2.4.3 淫羊藿苷对破骨细胞CAⅡ表达的影响 CAⅡ向破骨细胞膜上的V-ATP酶离子通道提供氢离子,在骨吸收过程中的酸化、氢离子分泌、疼痛信号传递、维持细胞内外酸碱平衡等方面起着至关重要的作用。研究结果显示,与对照组相比,模型组的TRAP和CAⅡ表达显著增加;与模型组相比,淫羊藿苷组则明显抑制了破骨细胞中CAⅡ和V-ATP酶的表达,见图3E。值得注意的是,CGRP和TRPV1表达表现出相似的趋势。这些结果表明淫羊藿苷具有阻碍卵巢切除小鼠疼痛的作用。"
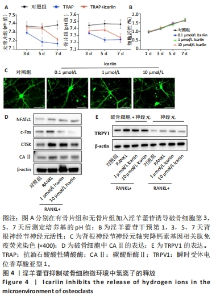
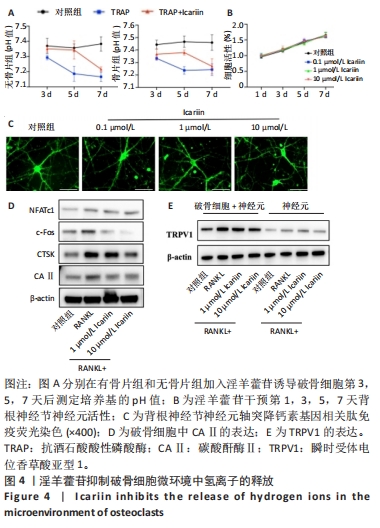
2.5 淫羊藿苷抑制破骨细胞微环境中氢离子的释放 2.5.1 淫羊藿苷对破骨细胞微环境pH值的影响 利用破骨细胞孵育的培养基作为条件培养基,模拟破骨细胞存活所需的酸性环境。破骨细胞活性增加导致骨吸收部位酸化。为了产生具有不同pH值的条件培养基,在无骨和含骨表面上用淫羊藿苷诱导破骨细胞。在诱导的第3,5,7天,测量了培养基的pH值,pH值保持在7.1-7.5之间。重要的是,淫羊藿苷干预组的pH值升高,见图4A。 2.5.2 淫羊藿苷对小鼠背根神经节神经元的增殖毒性作用 采用了CCK8实验测定不同浓度淫羊藿苷(0.1,1,10 μmol/L)在第1,3,5,7天的吸光度值,结果显示淫羊藿苷对背根神经节神经元的增殖无明显影响,见图4B。 2.5.3 淫羊藿苷对背根神经节功能的影响 CGRP是一种疼痛激活介质,在背根神经节激活后产生,参与外周和中枢神经源性疼痛信号的传递。为了评估不同H+浓度对背根神经节神经元轴突生长的影响,使用条件培养基共培养并进行CGRP免疫荧光染色,数据显示随着淫羊藿苷浓度的增加,背根神经节神经元轴突生长得到抑制,见图4C。 2.5.4 淫羊藿苷对CAⅡ和TRPV1表达的影响 研究表明,CAⅡ在H+分泌中发挥重要作用,维持破骨细胞内及周围稳定的酸碱微环境。Western blot分析清楚显示RANKL显著上调CAⅡ表达水平,而淫羊藿苷干预对CAⅡ表达的抑制作用呈浓度依赖性,见图4D。此外,TRPV1是参与痛觉的关键离子通道,在背根神经节神经元的兴奋和痛觉信号的传递中起着至关重要的作用。Western blot分析显示,随着淫羊藿苷浓度的增加,TRPV1表达水平显著降低,见图4E。"

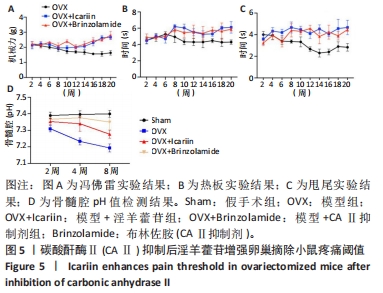
2.6 CAⅡ抑制后淫羊藿苷对卵巢摘除小鼠痛阈的增强作用 通过疼痛实验,观察到淫羊藿苷增加了小鼠的疼痛阈值。随后的组织分析显示,淫羊藿苷还抑制了CAⅡ的活性,该酶负责破骨细胞内H+的分泌。为了进一步研究H+分泌与疼痛的关系,特别是淫羊藿苷的作用,进行了额外的实验,见图5A-C。模型+CAⅡ抑制剂组小鼠疼痛阈值较模型组明显升高,说明CAⅡ介导的H+分泌与卵巢摘除小鼠疼痛阈值存在相关性;同样,模型+淫羊藿苷组小鼠也表现出类似的结果。这些发现提供了证据,表明淫羊藿苷抑制CAⅡ分泌H+,最终导致小鼠疼痛阈值提高。此外,与假手术组相比,模型组小鼠股骨pH值降低,表明卵巢切除术引起的改变导致股骨pH值降低;同时与模型组相比,模型+淫羊藿苷以及模型+CAⅡ抑制剂组小鼠股骨pH值显著升高,表明淫羊藿苷和CAⅡ抑制剂皆升高了股骨内的pH值,见图5D。"
| [1] LEMS WF, RATERMAN HG. Critical issues and current challenges in osteoporosis and fracture prevention. An overview of unmet needs. Ther Adv Musculoskelet Dis. 2017;9(12):299-316. [2] CIZZA G, PRIMMA S, CSAKO G. Depression as a risk factor for osteoporosis. Trends Endocrinol Metab. 2009;20(8):367-373. [3] KANIS JA, MCCLOSKEY EV, JOHANSSON H, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24(1):23-57. [4] TU KN, LIE JD, WAN CKV, et al. Osteoporosis: A Review of Treatment Options. P T. 2018;43(2):92-104. [5] BOYCE BF, XING L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473(2):139-146. [6] TEITELBAUM SL, ROSS FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4(8):638-649. [7] NOVACK DV. Role of NF-kappaB in the skeleton. Cell Res. 2011;21(1):169-182. [8] TAKAYANAGI H, KIM S, MATSUO K, et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002;416(6882): 744-749. [9] IKEBUCHI Y, AOKI S, HONMA M, et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature. 2018;561(7722):195-200. [10] KARTSOGIANNIS V, ZHOU H, HORWOOD NJ, et al. Localization of RANKL (receptor activator of NF kappa B ligand) mRNA and protein in skeletal and extraskeletal tissues. Bone. 1999;25(5):525-534. [11] LLOYD T, ANDON MB, ROLLINGS N, et al. Calcium supplementation and bone mineral density in adolescent girls. JAMA. 1993;270(7):841-744. [12] LIU Y, YANG H, XIONG J, et al. Icariin as an emerging candidate drug for anticancer treatment: Current status and perspective. Biomed Pharmacother. 2023;157:113991. [13] CHEN KM, GE BF, MA HP, et al. Icariin, a flavonoid from the herb Epimedium enhances the osteogenic differentiation of rat primary bone marrow stromal cells. Pharmazie. 2005;60(12):939-942. [14] ZHANG ZB, YANG QT. The testosterone mimetic properties of icariin. Asian J Androl. 2006;8(5):601-605. [15] LIAN F, ZHAO C, QU J, et al.Icariin attenuates titanium particle-induced inhibition of osteogenic differentiation and matrix mineralization via miR-21-5p. Cell Biol Int. 2018;42(8):931-939. [16] WANG ZQ, LI JL, SUN YL, et al. Chinese herbal medicine for osteoporosis: a systematic review of randomized controlled trails. Evid Based Complement Alternat Med. 2013;2013:356260. [17] OLESEN J, ASHINA M. Emerging migraine treatments and drug targets. Trends Pharmacol Sci. 2011;32(6):352-359. [18] ORITA S, INAGE K, SUZUKI M, et al. Pathomechanisms and management of osteoporotic pain with no traumatic evidence. Spine Surg Relat Res. 2017;1(3): 121-128. [19] CHOU YC, SHIH CC, LIN JG, et al. Low back pain associated with sociodemographic factors, lifestyle and osteoporosis: a population-based study. J Rehabil Med. 2013;45(1):76-80. [20] TSANG A, VON KORFF M, LEE S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9(10):883-891. [21] MANTYH PW.The neurobiology of skeletal pain. Eur J Neurosci. 2014;39(3):508-519. [22] MATTIA C, COLUZZI F, CELIDONIO L, et al. Bone pain mechanism in osteoporosis: a narrative review. Clin Cases Miner Bone Metab. 2016;13(2):97-100. [23] VELLUCCI R, TERENZI R, KANIS JA, et al. Understanding osteoporotic pain and its pharmacological treatment. Osteoporos Int. 2018;29(7):1477-1491. [24] CATALANO A, MARTINO G, MORABITO N, et al. Pain in Osteoporosis: From Pathophysiology to Therapeutic Approach. Drugs Aging. 2017;34(10):755-765. [25] PAOLUCCI T, SARACENI VM, PICCININI G. Management of chronic pain in osteoporosis: challenges and solutions. J Pain Res. 2016;9:177-186. [26] JULIUS D, BASBAUM AI. Molecular mechanisms of nociception. Nature. 2001; 413(6852):203-210. [27] BAMPS D, VRIENS J, DE HOON J, et al.TRP Channel Cooperation for Nociception: Therapeutic Opportunities. Annu Rev Pharmacol Toxicol. 2021;61:655-677. [28] HANSSON P. Translational aspects of central sensitization induced by primary afferent activity: what it is and what it is not. Pain. 2014;155(10):1932-1934. [29] FROST CØ, HANSEN RR, HEEGAARD AM. Bone pain: current and future treatments. Curr Opin Pharmacol. 2016;28:31-37. [30] ABBAS MA. Modulation of TRPV1 channel function by natural products in the treatment of pain. Chem Biol Interact. 2020;330:109178. [31] Aghazadeh Tabrizi M, BARALDI PG, BARALDI S, et al. Medicinal Chemistry, Pharmacology, and Clinical Implications of TRPV1 Receptor Antagonists. Med Res Rev. 2017;37(4):936-983. [32] Liu Y, Mi B, Lv H, et al. Shared KEGG pathways of icariin-targeted genes and osteoarthritis. J Cell Biochem. 2019;120(5):7741-7750. [33] Xue L, Jiang Y, Han T, et al. Comparative proteomic and metabolomic analysis reveal the antiosteoporotic molecular mechanism of icariin from Epimedium brevicornu maxim. J Ethnopharmacol. 2016;192:370-381. [34] Bellavia D, Dimarco E, Costa V, et al. Flavonoids in Bone Erosive Diseases: Perspectives in Osteoporosis Treatment. Trends Endocrinol Metab. 2021;32(2):76-94. [35] Xu Q, Chen G, Liu X, et al. Icariin inhibits RANKL-induced osteoclastogenesis via modulation of the NF-kappaB and MAPK signaling pathways. Biochem Biophys Res Commun. 2019;508(3):902-906. [36] Xu H, Zhou S, Qu R, et al. Icariin prevents oestrogen deficiency-induced alveolar bone loss through promoting osteogenesis via STAT3. Cell Prolif. 2020; 53(2):e12743. [37] Pfeilschifter J. Role of cytokines in postmenopausal bone loss. Curr Osteoporos Rep. 2003;1(2):53-58. [38] Wang Z, Wang D, Yang D, et al. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos Int. 2018;29(3):535-544. [39] Li J, Luo M, Wang S, et al. Icariin Ameliorates Lower Back Pain in Rats via Suppressing the Secretion of Cytokine-Induced Neutrophil Chemoatractant-1. Biomed Res Int. 2020;2020:4670604. [40] 中华医学会骨质疏松和骨矿盐疾病分会.原发性骨质疏松症诊疗指南(2017)[J].中国骨质疏松杂志,2019,25(3):281-309. [41] Rousselle AV, Heymann D. Osteoclastic acidification pathways during bone resorption. Bone. 2002;30(4):533-540. [42] Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355-384. [43] Mailhot B, Christin M, Tessandier N, et al. Neuronal interleukin-1 receptors mediate pain in chronic inflammatory diseases. J Exp Med. 2020;217(9):e20191430. [44] Kong WL, Peng YY, Peng BW. Modulation of neuroinflammation: Role and therapeutic potential of TRPV1 in the neuro-immune axis. Brain Behav Immun. 2017;64:354-366. |
| [1] | Guo Sutong, Feng Dehong, Guo Yu, Wang Ling, Ding Yujian, Liu Yi, Qian Zhengying, Li Mingyang. Construction and finite element analysis of normal and osteoporotic hip models [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1342-1346. |
| [2] | Wu Jing, Yao Yingce, Yang Xiaowei, Xue Boshi, Zhao Jianbin, Yang Chen, Luan Tianfeng, Zhou Zhipeng. Intervention of muscle strength training combined with neuromuscular electrical stimulation on lower limb function and biomechanical changes in patients with patellofemoral pain [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1365-1371. |
| [3] | Shan Jiaxin, Zhang Yilong, Wu Hongtao, Zhang Jiayuan, Li Anan, Liu Wengang, Xu Xuemeng, Zhao Chuanxi. Changes in muscle strength and pain in patients receiving Jianpi Yiqi Huoxue Formula after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1378-1382. |
| [4] | Li Xiaoqiang, Chen Wei, Li Mingyue, Shan Tianchi, Shen Wen. Value of preoperative quantitative ultrasound analysis of quadriceps femoris in predicting chronic post-surgical pain after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1388-1393. |
| [5] | Feng Tianxiao, Bu Hanmei, Wang Xu, Zhu Liguo, Wei Xu. Interpretation of key points of International Framework for Examination of the Cervical Region for potential of vascular pathologies of the neck prior to Orthopaedic Manual Therapy (OMT) Intervention: International IFOMPT Cervical Framework [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1420-1425. |
| [6] | Chen Zhiling, Huang Xuecheng, Pan Min, Huang Ying, Wu Yuntian. Evaluation of the relationship between neck and shoulder pain and scalene muscles based on shear wave elastography [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1265-1270. |
| [7] | Zhang Xiaoyun, Liu Hua, Chai Yuan, Chen Feng, Zeng Hao, Gao Zhengang, Huang Yourong. Effect of Yishen Gushu Formula on bone metabolic markers and clinical efficacyn in patients with osteoporosis of kidney deficiency and blood stasis type [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1155-1160. |
| [8] | Yue Yun, Wang Peipei, Yuan Zhaohe, He Shengcun, Jia Xusheng, Liu Qian, Li Zhantao, Fu Huiling, Song Fei, Jia Menghui. Effects of croton cream on JNK/p38 MAPK signaling pathway and neuronal apoptosis in cerebral ischemia-reperfusion injury rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1186-1192. |
| [9] | Dai Yuexing, Zheng Liqin, Wu Minhui, Li Zhihong, Li Shaobin, Zheng Desheng, Lin Ziling. Effect of vessel number on computational fluid dynamics in vascular networks [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1206-1210. |
| [10] | Han Bing, Liu Hongbin, Wang Hehong, Zhao Hanqing, Zhao Riguang, Sun Yiyan, Zhang Yu. Correlation between lower limb alignment and risk factors of patellofemoral pain syndrome in young men [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1211-1216. |
| [11] | Li Longyang, Zhang Songjiang, Zhao Xianmin, Zhou Chunguang, Gao Jianfeng. Electroacupuncture intervention on the proliferation and differentiation of hippocampal neurons and oligodendrocytes in Alzheimer’s disease model mice [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1029-1035. |
| [12] | Zhang Min, Peng Jing, Zhang Qiang, Chen Dewang. Mechanical properties of L3/4 laminar decompression and intervertebral fusion in elderly osteoporosis patients analyzed by finite element method [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 847-851. |
| [13] | Xue Xiaofeng, Wei Yongkang, Qiao Xiaohong, Du Yuyong, Niu Jianjun, Ren Lixin, Yang Huifeng, Zhang Zhimin, Guo Yuan, Chen Weiyi. Finite element analysis of osteoporosis in proximal femur after cannulated screw fixation for femoral neck fracture [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 862-867. |
| [14] | Kaiyisaier•Abudukelimu, Maimaitimin•Abulimiti, Li Lei, Yang Xiaokai, Zhang Yukun, Liu Shuai. Effect of lumbar CT values in the diagnosis of osteoporosis in women patients with lumbar degenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 945-949. |
| [15] | Wang Liping, Lian Tianxing, Hu Yongrong, Yang Hongsheng, Zeng Zhimou, Liu Hao, Qu Bo. HU value of chest CT vertebral body in the opportunistic screening of type 2 diabetes mellitus osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 950-954. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||