Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (16): 2519-2526.doi: 10.12307/2024.302
Previous Articles Next Articles
Effects of transcranial magneto-acoustical stimulation on beta oscillations in neural circuits of healthy and Parkinson’s disease rats
Zhang Shuai1, 2, 3, You Shengnan1, 2, 3, Du Wenjing1, 2, 3, Wang Lei1, 2, 3, Xu Guizhi1, 2, 3
- 1State Key Laboratory of Reliability and Intelligence of Electrical Equipment, 2Hebei key Laboratory of Bioelectromagnetism and Neural Engineering, 3Tianjin Key Laboratory of Bioelectromagnetic Technology and Intelligent Healthy, Hebei University of Technology, Tianjin 300401, China
-
Received:2022-12-23Accepted:2023-04-12Online:2024-06-08Published:2023-07-29 -
Contact:Zhang Shuai, State Key Laboratory of Reliability and Intelligence of Electrical Equipment, Hebei key Laboratory of Bioelectromagnetism and Neural Engineering, Tianjin Key Laboratory of Bioelectromagnetic Technology and Intelligent Healthy, Hebei University of Technology, Tianjin 300401, China -
About author:Zhang Shuai, PhD, Professor, State Key Laboratory of Reliability and Intelligence of Electrical Equipment, Hebei key Laboratory of Bioelectromagnetism and Neural Engineering, Tianjin Key Laboratory of Bioelectromagnetic Technology and Intelligent Healthy, Hebei University of Technology, Tianjin 300401, China -
Supported by:National Natural Science Foundation of China, No. 51877069 (to ZS); Natural Science Foundation of Hebei Province, No. E2021202184 (to ZS)
CLC Number:
Cite this article
Zhang Shuai, You Shengnan, Du Wenjing, Wang Lei, Xu Guizhi. Effects of transcranial magneto-acoustical stimulation on beta oscillations in neural circuits of healthy and Parkinson’s disease rats[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(16): 2519-2526.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
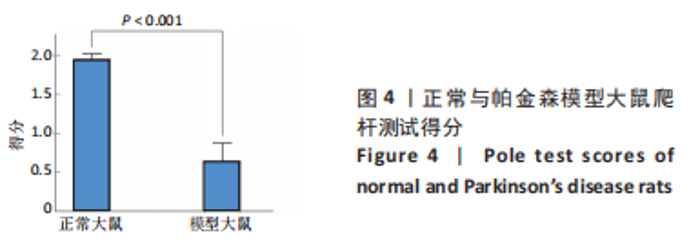
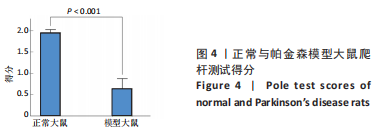
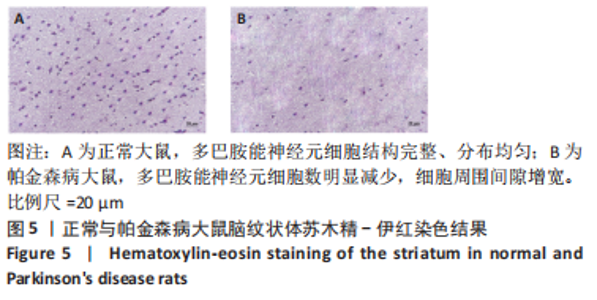
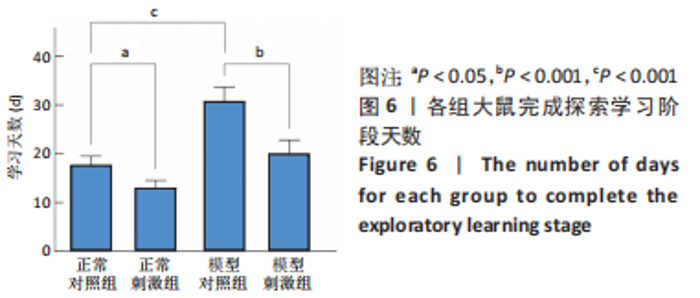
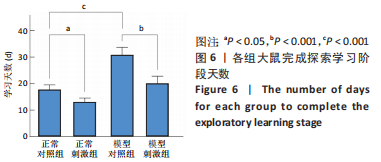
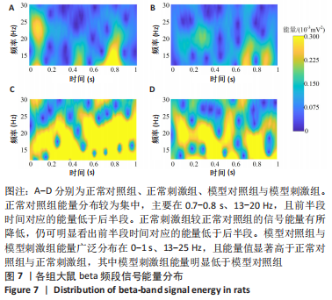
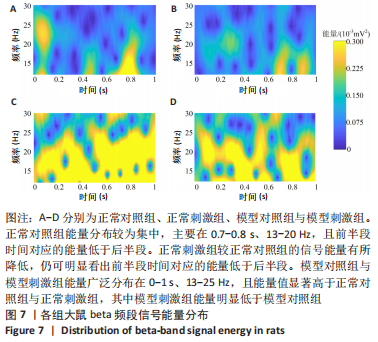
正常对照组能量分布较为集中,主要在0.7-0.8 s、13-20 Hz,且前半段时间对应的能量低于后半段。正常刺激组较正常对照组的信号能量有所降低,仍可明显看出前半段时间对应的能量低于后半段。而模型对照组与模型刺激组能量广泛分布在0-1 s、13-25 Hz,且能量值显著高于正常对照组与正常刺激组,其中模型刺激组能量明显低于模型对照组。由此说明,经颅磁声电刺激降低了正常刺激组与模型刺激组大鼠的beta振荡。此外,正常对照组存在能量集中分布在后半段时间的情况,对应于大鼠做出选择后即将到达目标位置,这可能与准备停止运动过程有关。 2.2 建模仿真实验结果 2.2.1 健康大鼠仿真结果 如图8所示,横坐标为beta频段的频率,纵坐标为功率谱密度值,曲线上每点表示苍白球内侧部神经元在该放电节律下的平均功率谱密度值。正常对照组的功率谱密度值在beta频段上的变化范围为10-31 dB,低于此区间可能导致大鼠情绪紧张、心率加快,高于此区间可能导致大鼠运动功能减退和强直[41]。"
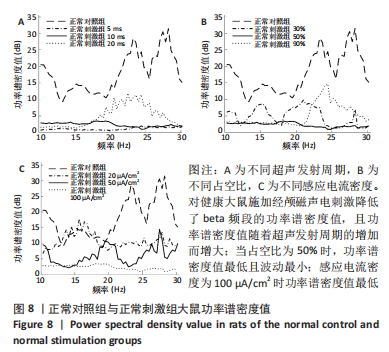
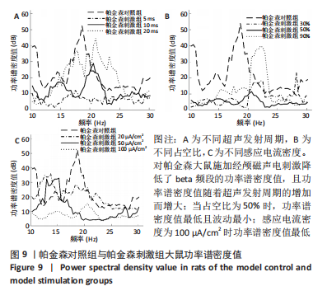
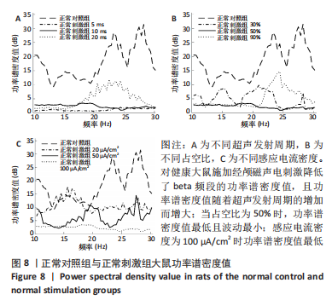
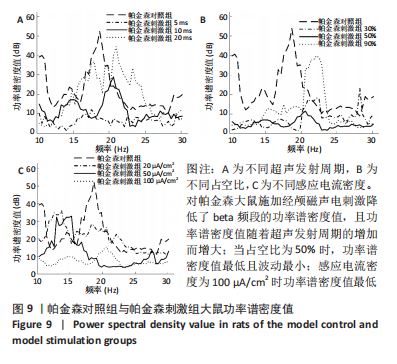
施加经颅磁声电刺激后,普遍存在功率谱密度值下降的现象,如图8A所示,当超声发射占空比为50%、感应电流密度为100 μA/cm2时,调节超声发射周期为5,10,20 ms时发现,正常刺激组发射周期为5 ms时的功率谱密度值最低、20 ms时的功率谱密度值最高,但低于正常对照组。说明对健康大鼠施加经颅磁声电刺激降低了beta频段的功率谱密度值,且功率谱密度值随着超声发射周期的增加而增大。 调节超声发射周期改变了神经元放电节律,推测与超声发射周期和神经元动作电位的发放率呈正比关系有关[6],发射周期的增大导致神经元动作电位发放率的增大,进而出现更多较高频的beta节律振荡。如图8B所示,当超声发射周期为10 ms、感应电流密度为100 μA/cm2时,调节正常刺激组超声发射的占空比为30%,50%,90%时发现,当占空比为50%时,功率谱密度值最低且波动最小,占空比为30%和90%时的功率谱密度幅值均高于50%,但低于正常对照组。由此看出,当占空比接近50%时,经颅磁声电刺激抑制beta振荡的效果最好,这可能与占空比可影响经颅磁声电刺激的去同步效果有关,神经系统对调制波的占空比具有选择性,系统的同步状态分布在占空比为10%-30%和80%-90%之间,去同步状态集中于40%-70%[42],在去同步状态中beta频段振幅降低,导致功率谱密度值低于其他占空比。 如图8C所示,当超声发射周期为10 ms、占空比为50%时,改变正常刺激组感应电流密度为20,50,100 μA/cm2时发现,感应电流密度为100 μA/cm2时功率谱密度值最低,当感应电流密度为50 μA/cm2时,功率谱密度值有所提高,但低于正常对照组,当感应电流密度进一步降低为20 μA/cm2时功率谱密度值进一步提高。说明改变经颅磁声电刺激的感应电流密度可调节beta频段的功率谱密度值,二者之间呈现负相关,推测是因为感应电流密度的提高增大了皮质神经元的突触输入,皮质基底神经节直接通路与间接通路共同发挥作用,抑制了大鼠皮质-基底神经节环路中的beta振荡。 2.2.2 帕金森病大鼠仿真实验结果 如图9所示,过度的beta振荡是帕金森病的主要特征,由于帕金森病大鼠黑质纹状体内多巴胺显著减少,导致直接通路中含有D1受体的纹状体神经元的兴奋性降低、间接通路中含有D2类受体的纹状体神经元的抑制性降低,进一步导致纹状体对苍白球内侧部的抑制削弱,使得健康状态的平衡被打破,进而促进帕金森病疾病的表现,使beta频段功率谱密度值主要在10-52 dB范围内波动,明显高于健康大鼠。"
| [1] UNDERWOOD E. Short-Circuiting depression. Science. 2013;342(6158): 548-551. [2] SHEN H. Tuning the brain. Nature. 2014;507(7492):290-293. [3] BIKSON M, BESTMANN S, EDWARDS D. Transcranial Devices are not playthings. Nature, 2013;501:7466. [4] ZHANG S, CUI K, ZHANG X, et al. Effect of transcranial ultrasonic-magnetic stimulation on two types of neural firing behaviors in modified izhikevich model. IEEE T Magn. 2018;54(3):1-4. [5] NORTON SJ. Can ultrasound be used to stimulate nerve tissue? Biomed Eng Online. 2003;2(1):6-24. [6] 袁毅,陈玉东,闫佳庆,等.经颅霍尔效应刺激作用下神经元系统放电节律的理论研究[J].中国生物医学工程学报,2016,35(2):247-251. [7] 刘世坤,张鑫山,周晓青,等.经颅磁声耦合电刺激技术应用于小鼠的实验研究[J].生物医学工程研究,2018,37(1):11-15. [8] ZHANG YQ, ZHANG M, LING ZC, et al. The influence of transcranial magnetoacoustic stimulation parameters on the basal ganglia-thalamus neural network in Parkinson ‘s disease. Front Nrurosci. 2021;15:761720. [9] 张帅,党君武,焦立鹏,等.经颅磁声电刺激对大鼠工作记忆局部场电位gamma节律的影响[J].中国生物医学工程学报,2021,40(5): 540-549. [10] 党君武,张帅,由胜男,等.经颅磁声电刺激影响大鼠工作记忆中局部场电位的相位幅值耦合分析[J]. 生物医学工程学杂志,2022, 39(2):267-275. [11] FOFFANI G, ALEGRE M. Brain oscillations and Parkinson disease. Handb Clin Neurol. 2022;184:259-271. [12] GUERRA A, COLELLA D, GIANGROSSO M, et al. Driving motor cortex oscillations modulates bradykinesia in Parkinson ’s disease. Brain. 2022;145(1):224-236. [13] HAUMESSER JK, BECK MH, PELLEGRINI F, et al. Subthalamic beta oscillations correlate with dopaminergic degeneration in experimental parkinsonism. Exp Neurol. 2021;335:113513. [14] SU F, CHEN M, ZU L, et al. Model-based closed-loop suppression of Parkinsonian beta band oscillations through origin analysis. IEEE T Neur Sys Reh. 2021;29:450-457. [15] YU Y, HAN F, WANG Q. Exploring phase-amplitude coupling from primary motor cortex-basal ganglia-thalamus network model. Neural Netw. 2022;153:130-141. [16] ASADI A, MADADI AM, VAHABIE AH, et al. The Origin of Abnormal Beta Oscillations in the Parkinsonian Corticobasal Ganglia Circuits. Parkinsons Dis. 2022;2022:7524066. [17] MURALIDHARAN V, ARON AR. Behavioral induction of a high beta state in sensorimotor cortex leads to movement slowing. J Cogn Neurosci. 2021;33(7):1311-1328. [18] CROSS KA, MALEKMOHAMMADI M, CHOI JW, et al. Movement-related changes in pallidocortical synchrony differentiate action execution and observation in humans. Clin Neurophysiol. 2021;132(8):1990-2001. [19] WEI W, RUBIN JE, WANG XJ. Role of the indirect pathway of the basal ganglia in perceptual decision making. J. Neurosci. 2015;35(9):4052-4064. [20] ZHANG Y, CHEN Y, BRESSLER SL, et al. Response preparation and inhibition: the role of the cortical sensorimotor beta rhythm. Neuroscience. 2008;156(1):238-246. [21] LO CC, WANG XJ. Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat Neurosci. 2006;9(7):956-963. [22] 闫睿,魏婧,贾军,等.皮层-基底神经节环路beta振荡与帕金森病运动障碍的关系[J].生理科学进展,2022,53(4):254-258. [23] CAO C, LI D, ZHAN S, et al. L-dopa treatment increases oscillatory power in the motor cortex of Parkinson ‘s disease patients. J Neurochem. 2020;26:102255. [24] LUO F, KISS ZHT. Cholinergic mechanisms of high-frequency stimulation in entopeduncular nucleus. J Neurosci. 2016;115(1):60-67. [25] ZHU GY, GENG XY, ZHANG RL, et al. Deep brain stimulation modulates pallidal and subthalamic neural oscillations in Tourette ‘s syndrome. Brain Behav. 2019;9(12):e01450. [26] LITTLE S, BROWN P. The functional role of beta oscillations in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:44-48. [27] FASANO A, AQUINO CC, KRAUSS JK, et al. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat Rev Nephrol. 2015;11(2): 98-110. [28] MCKIM DB, WEBER MD, NIRAULA A, et al. Microglial recruitment of IL-1 beta-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry. 2018;23(6):1421-1431. [29] FUKUSHIMA K, HASHIMOTO T, YAKO T, et al. Deep brain stimulation on neuronal intranuclear inclusion disease-related tremor: a double-edged impact? Mov Disord Clin Pract. 2022;9(7):983-986. [30] 曾朝蓉,岳冬,孙伟,等.亚硒酸钠对帕金森模型大鼠运动功能及脑黑质抗氧化能力的影响[J].中华行为医学与脑科学杂志,2020, 29(5):413-418. [31] QUIROGA-VARELA A, WALTERS JR, BRAZHNIK E, et al. What basal ganglia changes underlie the parkinsonian state? The significance of neuronal oscillatory activity. Neurobiol Dis. 2013;58:242-248. [32] MAILMAN RB, YANG Y, HUANG X. D1, not D2, dopamine receptor activation dramatically improves MPTP-induced parkinsonism unresponsive to levodopa. Eur J Pharmacol. 2021;892:173760. [33] 曾朝蓉,岳冬,孙伟,等.MPTP 诱导帕金森模型大鼠的血液学及临床病理评价[J].成都医学院学报,2020,15(1):23-27. [34] 张帅,武健康,党君武,等.经颅磁声电刺激下不同类型 Izhikevich 神经元电耦合模型放电特性研究[J].生命科学仪器,2021,19(2):67-75. [35] 张帅,史勋,尹宁,等.基于HH神经元模型的经颅磁声刺激对神经元放电活动的影响[J].高电压技术,2019,45(4):1124-1130. [36] 张帅,高昕宇,周振宇,等.基于GrC模型的经颅磁声电刺激对神经元放电活动的影响[J].电工技术学报,2019,34(17):3572-3580. [37] KUMARAVELU K, BROCKER DT, GRILL WM. A biophysical model of the cortex-basal ganglia-thalamus network in the 6-OHDA lesioned rat model of Parkinson ’s disease. J Comput Neurosci. 2016;40(2):207-229. [38] TOPALIDOU M, KASE D, BORAUD T, et al. A computational model of dual competition between the basal ganglia and the cortex. eNeuro. 2018;5(6):1-17. [39] PAULY MG, BARLAGE M, HAMAMI F. Subthalamic nucleus conditioning reduces premotor-motor interaction in Parkinson ‘s disease. Parkinsonism Relat Disord. 2022;96:6-12. [40] GARRETT M, BRADY A, ALEXEY K, et al. Basal ganglia role in learning rewarded actions and executing previously learned choices: healthy and diseased states. PLoS One. 2020;15(2):e0228081. [41] DARROW CW. The electroencephalogram and psychophysiological regulation in the brain. Am J Geriat Psychiat. 1946;102(6):791-798. [42] 袁毅,孙红宝,陈玉东,等.经颅磁声刺激作用下耦合神经元的去同步研究[J].中国生物医学工程学报,2017,36(4):502-506. [43] DOYLE GAYNOR LMF, KÜHN AA, DILEONE M, et al. Suppression of beta oscillations in the subthalamic nucleus following cortical stimulation in humans. Eur J Neurosci. 2008;28(8):1686-1695. [44] DELONG MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13(7):281-285. [45] CHEN CC, HSU YT, CHAN HL, et al. Complexity of subthalamic 13–35 Hz oscillatory activity directly correlates with clinical impairment in patients with Parkinson ‘s disease. Exp Neurol. 2010;224(1):234-240. [46] SAMIEE K, KOVACS P, GABBOUJ M. Epileptic seizure classification of EEG time-series using rational discrete short-time fourier transform. IEEE T Bio-Med Eng. 2015;62(2):541-552. [47] FLEMING JE, ORŁOWSKI J, LOWERY MM, et al. Self-tuning deep brain stimulation controller for suppression of beta oscillations: analytical derivation and numerical validation. Front Neurosci. 2020;14:639. [48] BROWN GL, BROWN MT. Transcranial electrical stimulation in neurological disease. Neural Regen Res. 2022;17(10):2221-2222 |
| [1] | Qi Xue, Li Jiahui, Zhu Yuanfeng, Yu Lu, Wang Peng. Abnormal modification of alpha-synuclein and its mechanism in Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1301-1306. |
| [2] | Ma Xinran, Liu Xinhao, Li Yujia, Luo Kailiang, Ma Shujie, Hu Jun. Effect of treadmill exercise on the structure and diversity of intestinal microflora in rats with Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(14): 2227-2233. |
| [3] | Li Jiahui, Qi Xue, Zhu Yuanfeng, Yu Lu, Liu Lifeng, Wang Peng. Protective effect of C2 ceramide on dopaminergic neurons in a mouse model of Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(11): 1653-1659. |
| [4] | Yang Yulin, Chang Wanpeng, Ding Jiangtao, Xu Hongli, Wu Xiao, Xiao Boheng, Ma Lihong. Transcranial direct current stimulation at different targets for Parkinson’s disease: a network Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(11): 1797-1804. |
| [5] | Qi Xue, Li Jiahui, Zhu Yuanfeng, Yu Lu, Wang Peng. Phosphorylation modification of alpha synuclein in serum of patients with Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(35): 5577-5582. |
| [6] | Zheng Xiaohan, Feng Xiaoli, Hu Lan, Gao Shijun, Wei Yanzhao, Huang Ting, Sun Shengtong, Wei Xufang, Wang Tan, Zhao Zhenqiang. Macrophage migration inhibitory factor promotes the differentiation of human embryonic stem cells into ventral midbrain dopaminergic neural progenitor cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5348-5356. |
| [7] | Wang Yiying, Li Ruiqing, Li Jingwen, Mei Jinjin, Zhang Jianyun, Zhang Lihong, Fan Yongfu, Guo Jian. Exosome-mediated cellular communication: a potential biomarker for Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(24): 3883-3891. |
| [8] | Chen Shijian, Li Ge, Zhang Yu, Guan Yalun, Li Xuejiao, Liu Shuhua, Li Yongchao, Li Yunfeng, Gao Jinfeng, Wei Xiaoyue, Zhao Yuhong. Comparison and evaluation of MPTP-induced subacute and chronic models of Parkinson’s disease in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1247-1252. |
| [9] | Zhu Chan, Han Xuke, Yao Chengjiao, Zhang Qiang, Liu Jing, Shao Ming. Acupuncture for Parkinson’s disease: an insight into the action mechanism in animal experiments [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1272-1277. |
| [10] | Wei Yufan, Fan Feiyan, Li Shuangli, Zhang Yunke. Future of exosomes and mesenchymal stem cell-derived exosomes in the diagnosis and treatment of Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 4076-4083. |
| [11] | Wang Hujun, Wang Yingpeng, Fang Boyan, Jin Zhaohui, Qi Lin, Zhang Qiaorong, Wang Congxiao, Qie Shuyan. Effect of forearm weight-bearing on spatiotemporal parameters and joint angles of the lower limbs in patients with Parkinson’s disease during walking [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(15): 2307-2311. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||