Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (34): 5497-5504.doi: 10.12307/2023.808
Previous Articles Next Articles
Biosafety evaluation of poly(lactic acid)/nano-hydroxyapatite composite microspheres
Zhao Kang1, Lin Si1, Ge Xiaotian1, Du Xinrui1, Han Yingchao1, 2
- 1State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, Hubei Province, China; 2Foshan Xianhu Laboratory, Foshan 528200, Guangdong Province, China
-
Received:2022-09-19Accepted:2022-11-21Online:2023-12-08Published:2023-04-22 -
Contact:Han Yingchao, Researcher, Doctoral supervisor, State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, Hubei Province, China; Foshan Xianhu Laboratory, Foshan 528200, Guangdong Province, China -
About author:Zhao Kang, Master candidate, State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, Hubei Province, China -
Supported by:National Key Research & Development Program, No. 2016YFB1101302 (to HYC); Foshan Xianhu Laboratory Open Fund, No. XHT2020-008 (to HYC)
CLC Number:
Cite this article
Zhao Kang, Lin Si, Ge Xiaotian, Du Xinrui, Han Yingchao. Biosafety evaluation of poly(lactic acid)/nano-hydroxyapatite composite microspheres[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5497-5504.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
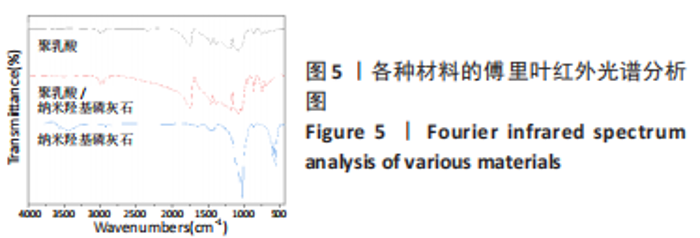
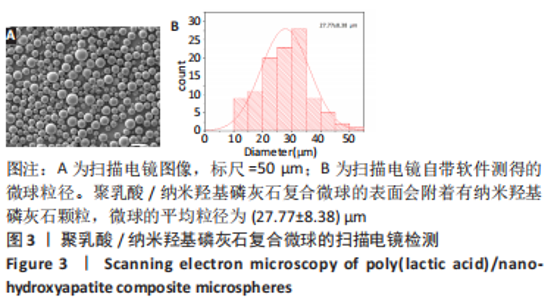
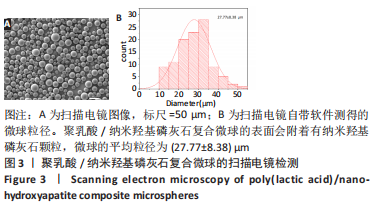
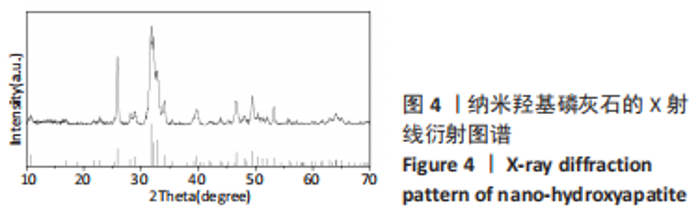
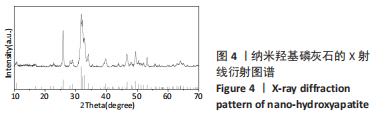
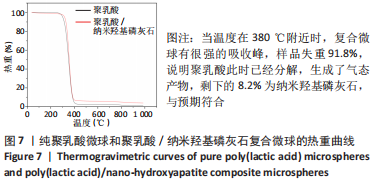
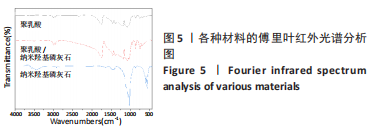
图5为纯聚乳酸微球、聚乳酸/纳米羟基磷灰石复合微球和纳米羟基磷灰石的傅里叶红外光谱分析图。在图中可以看到,纯聚乳酸微球在1 758 cm-1处的强吸收峰为C=O的伸缩振动峰;1 037,1 077,1 134,1 183 cm-1处为C-O-O的伸缩振动峰,则证明了酯基的存在;1 277 cm-1的吸收峰对应CH-OH;1 386,1 459,2 881,2 946 cm-1对应-CH3的振动峰;1 369,2 881 cm-1归属于-CH的弯曲和伸缩振动峰;3 655 cm-1则是聚乳酸端羟基(-OH)的吸收峰。由此可以证明纯聚乳酸的微球成分为聚乳酸,且在制备过程中未发生化学变化。将聚乳酸/纳米羟基磷灰石复合微球与纯聚乳酸微球、纳米羟基磷灰石的红外光谱图进行对比,发现聚乳酸1 758 cm-1处C=O和1 077 cm-1处C-O-O的伸缩振动峰强度增强,端羟基的吸收峰与纳米羟基磷灰石的吸附水峰合并成3 436 cm-1处弱宽峰,指纹区1 039,602,565 cm-1处PO43-的特征吸收峰证明了纳米羟基磷灰石的存在。纳米羟基磷灰石羟基的伸缩振动峰未发生偏移,说明纳米羟基磷灰石与聚乳酸间无氢键生成,进一步说明了纳米羟基磷灰石和聚乳酸只是单纯的物理混合,两者间没有发生任何化学变化。"
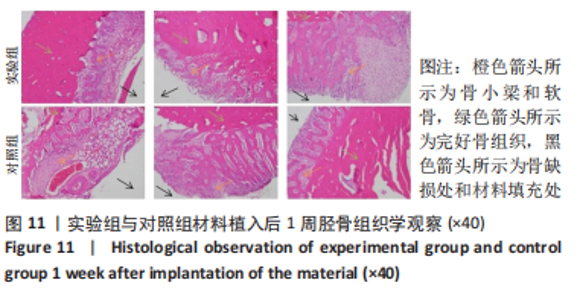
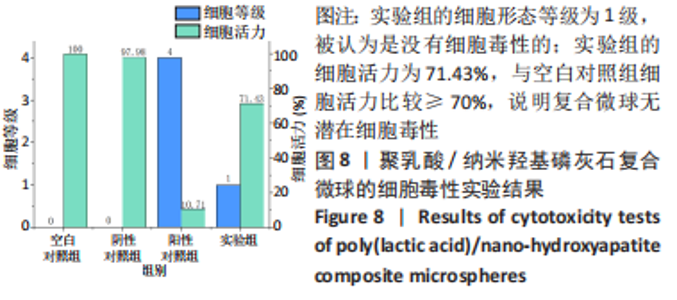
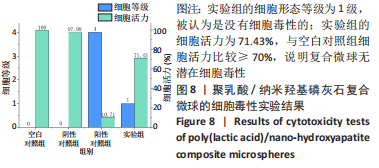
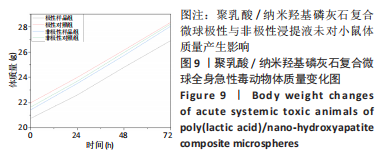
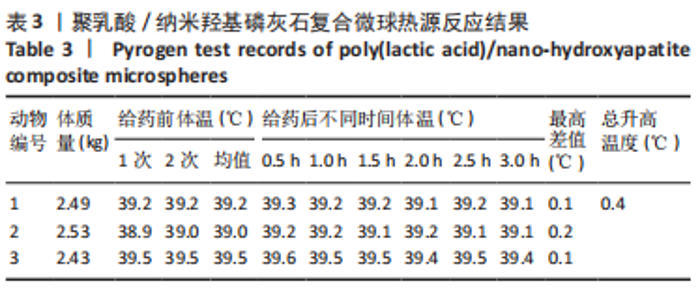
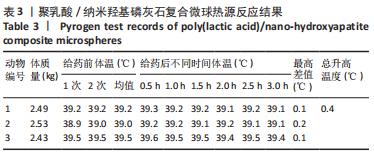
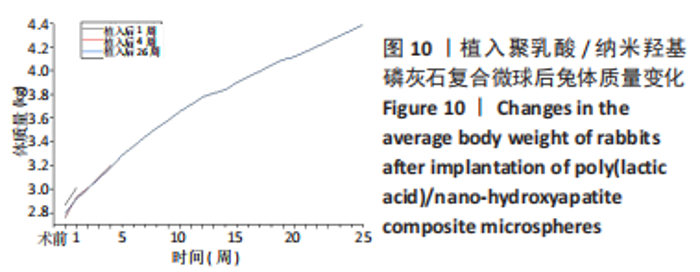
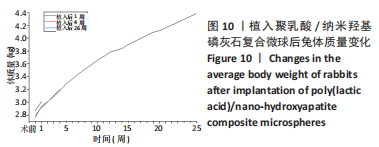
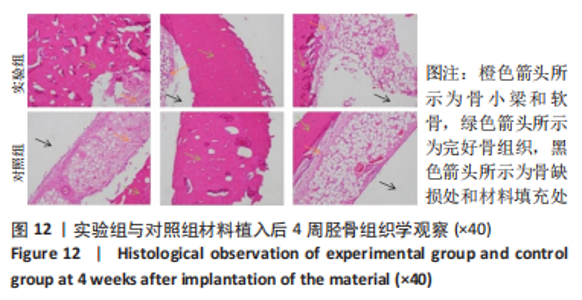
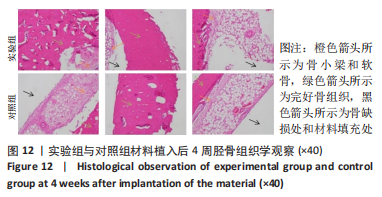
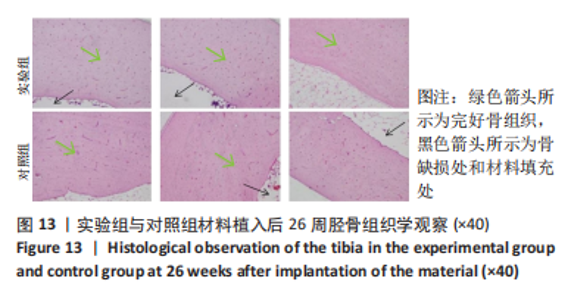
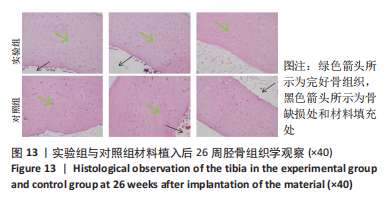
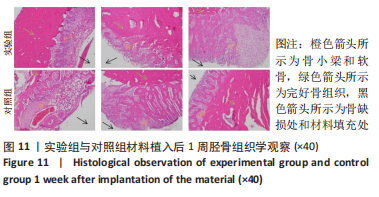
材料植入后1周组织学观察结果如图11所示,实验组与对照组植入缺损部位骨外膜可见较规则的骨小梁和软骨形成,周围残留少量结缔组织,缺损部位骨内侧可见骨小梁和软骨形成,其间可见纤维结缔组织和炎症反应。材料植入后4周组织学观察结果如图12所示,实验组植入缺损部位骨外膜可见较规则的骨小梁和软骨形成,部分植入缺损部位骨外膜可见编织骨形成,周围残留少量纤维组织,缺损部位骨内侧可见纤维结缔组织和骨小梁形成,并伴有炎症反应;对照组植入缺损部位骨外膜可见较规则的骨小梁和编织骨形成,周围残留少量纤维组织,缺损部位骨内侧可见纤维结缔组织、骨小梁和编织骨形成,并伴有炎症反应。材料植入后26周组织学观察结果如图13所示,两组植入缺损部位可见薄层纤维包膜,缺损部位新密质骨形成,并可见轮状哈佛氏管内血管,骨内侧边缘不规则,骨厚度增加,部分缺损部位可见新的小骨髓腔,骨内侧边缘不规则。组织学半定量评分结果显示,实验组植入后1,4,26周刺激评分为1.42,0.00,0.00,刺激等级均为无刺激。"
| [1] XIE C, YE JC, LIANG RJ, et al. Advanced Strategies of Biomimetic Tissue-Engineered Grafts for Bone Regeneration. Adv Healthcare Mater. 2021;10(14):e2100408. [2] LEE K, GOODMAN SB. Current state and future of joint replacements in the hip and knee. Expert Rev Med Devices. 2008;5(3):383-393. [3] NARAYANAN G, VERNEKAR VN, KUYINU EL, et al. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv Drug Delivery Rev. 2016;107:247-276. [4] GANGAPURWALA G, VOLLRATH A, DE SAN LUIS A, et al. PLA/PLGA-Based Drug Delivery Systems Produced with Supercritical CO2-A Green Future for Particle Formulation? Pharmaceutics. 2020;12(11):1118. [5] SANTOS C, LUKLINSKA ZB, CLARKE RL, et al. Hydroxyapatite as a filler for dental composite materials: mechanical properties and in vitro bioactivity of composites. J Mater Sci-Mater M. 2001;12(7):565-573. [6] DOROZHKIN SV. Bioceramics of calcium orthophosphates. Biomaterials. 2010; 31(7):1465-1485. [7] WANG QF, YAN JH, YANG JL, et al. Nanomaterials promise better bone repair. Mater Today. 2016;19(8):451-463. [8] 刘晓南,张道海,李刚,等.聚乳酸/纳米羟基磷灰石/脱胶蚕丝纤维多孔支架的制备与性能[J].工程塑料应用,2019,47(12):14-20. [9] FU Z, CUI JJ, ZHAO B, et al. An overview of polyester/hydroxyapatite composites for bone tissue repairing. J Orthop. Transl. 2021;28:118-130. [10] 王靖,刘昌胜.材料生物学——骨修复材料的机遇与挑战[J].中国材料进展,2019,38(4):359-364. [11] 秦宇星,任前贵,沈佩锋.组织工程骨技术治疗骨缺损的优越性[J].中国组织工程研究,2020,24(24):3877-3882. [12] KACAREVIC ZP, RIDER P, ALKILDANI S, et al. An introduction to bone tissue engineering. Int J Artif Organs. 2020;43(2):69-86. [13] 马士卿,王洁,高平,等.生物降解微球在骨组织再生中的生物学优势[J].中国组织工程研究,2021,25(34):5517-5522. [14] 张瑶.缓释微球制剂的研究进展[J].医药导报,2004(11):843-844. [15] YAN D, ZENG B, HAN YC, et al. Preparation and laser powder bed fusion of composite microspheres consisting of poly(lactic acid) and nano-hydroxyapatite. Addit Manuf. 2020;34:101345. [16] ZHANG BQ, WANG L, SONG P, et al. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater Des. 2021;201: 109490. [17] YUAN XT, LIN S, ZHAO K, et al. Emulsion-ultrasonic spray method to prepare polylactic acid microspheres. Mater Lett. 2022;309:131461.1-131461.5. [18] NEVADO P, LOPERA A, BEZZON V, et al. Preparation and in vitro evaluation of PLA/biphasic calcium phosphate filaments used for fused deposition modelling of scaffolds. Mat SCI ENG C-Mater. 2020;114:111013. [19] LIU SQ, ZHENG YY, LIU RL, et al. Preparation and characterization of a novel polylactic acid/hydroxyapatite composite scaffold with biomimetic micro-nanofibrous porous structure. J Mater Sci-Mater M. 2020;31(8):74. [20] 尹浩月.聚乳酸/纳米羟基磷灰石复合支架材料的制备与研究[D].唐山:华北理工大学,2019. [21] 马喜峰.聚乳酸的改性及应用研究进展[J].化学与粘合,2021,43(6):473-476+484. [22] 刘文涛,徐冠桦,段瑞侠,等.聚乳酸改性与应用研究综述[J].包装学报, 2021,13(2):3-13+19. [23] SU Y, ZHANG BL, SUN RW, et al. PLGA-based biodegradable microspheres in drug delivery: recent advances in research and application. Drug Deliv. 2021;28(1): 1397-1418. [24] KANG SW, YANG HS, SEO SW, et al. Apatite-coated poly(lactic-co-glycolic acid) microspheres as an injectable scaffold for bone tissue engineering. J Biomed Mater Res Part A. 2008;85A(3):747-756. [25] WEE CY, YANG ZJ, THIAN ES. Past, present and future development of microspheres for bone tissue regeneration: a review. Mater Technol. 2021;36(6):364-374. [26] LEE CSD, MOYER HR, GITTENS RA, et al. Regulating in vivo calcification of alginate microbeads. Biomaterials. 2010;31(18):4926-4934. [27] MENG D, DONG LM, WEN Y, et al. Effects of adding resorbable chitosan microspheres to calcium phosphate cements for bone regeneration. Mat Sci Eng C-Mater. 2015;47:266-272. [28] ZHANG R, LIN MY, WANG CX, et al. Bioinspired fabrication of EDC-crosslinked gelatin/nanohydroxyapatite injectable microspheres for bone repair. Int J Polym Mater Polym Biomater. 2022;309:131461.1-131461.5. [29] SONG WZ, WANG DZ, GUO M, et al. Assessment of nano-hydroxyapatite and poly (lactide-co-glycolide) nanocomposite microspheres fabricated by novel airflow shearing technique for in vivo bone repair. Mater Sci Eng C Mater Biol Appl. 2021;128:112299. [30] 张伟忠,李磊,何贺,等.纳米纤维大孔支架制备技术在骨组织工程研究中的应用与意义[J].中国组织工程研究,2020,24(28):4437-4444. [31] LIU YK, WANG GC, CAI YR, et al. In vitro effects of nanophase hydroxyapatite particles on proliferation and osteogenic differentiation of bone marrow-derived mesenchymal stem cells. J Biomed Mater Res Part A. 2009;90A(4):1083-1091. [32] SATPATHY A, PAL A, SENGUPTA S, et al. Bioactive Nano-Hydroxyapatite Doped Electrospun PVA-Chitosan Composite Nanofibers for Bone Tissue Engineering Applications. J Indian Inst Sci. 2019;99(3):289-302. [33] JIANG WS, RUTHERFORD D, VUONG T, et al. Nanomaterials for treating cardiovascular diseases: A review. Bioact Mater. 2017;2(4):185-198. [34] BOOS AM, WEIGAND A, BRODBECK R, et al. The potential role of telocytes in Tissue Engineering and Regenerative Medicine. Semin Cell Dev Biol. 2016;55:70-78. [35] HARMS C, HELMS K, TASCHNER T, et al. Osteogenic capacity of nanocrystalline bone cement in a weight-bearing defect at the ovine tibial metaphysis. Int J Nanomed. 2012;7:2883-2889. [36] SCHÜTZ CA, JUILLERAT-JEANNERET L, MUELLER H, et al. Therapeutic nanoparticles in clinics and under clinical evaluation. Nanomedicine. 2013;8:449-467. [37] ELSAESSER A, HOWARD CV. Toxicology of nanoparticles. Adv Drug Delivery Rev. 2012;64(2):129-137. [38] BAAN RA. Carcinogenic hazards from inhaled carbon black, titanium dioxide, and talc not containing asbestos or asbestiform fibers: recent evaluations by an IARC Monographs Working Group. Inhalation Toxicol. 2007;19(Suppl. 1):213-228. [39] SARGENT LM, PORTER DW, STASKA LM, et al. Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol. 2014;11:3. [40] JOHANNSEN M, GNEVECKOW U, TAYMOORIAN K, et al. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: results of a prospective phase I trial. Int J Hyperthermia. 2007; 23:315-323. [41] ZHAO JS, CASTRANOVA V. Toxicology of Nanomaterials used in Nanomedicine. J Toxicol Env Heal B. 2011;14(8):593-632. |
| [1] | Xu Shicai, Ma Fei, Tang Chao, Liao Yehui, Tang Qiang, Chen Shiyu, Li Yang, Zhou Jiajun, Wang Qing, Zhong Dejun. Effect of nano-hydroxyapatite/polyamide-66 intervertebral fusion cage shape on the efficacy of anterior cervical discectomy and fusion [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4830-4835. |
| [2] | Yan Nan, Wu Xuanjing, Wang Ziqi, Wu Jing, Zhao Tongtong, Xue Yan, Zhao Weijie, Wang Lijie, Wu Zimei, Zhang Wenqing, Li Jing. Synthesis and drug loading and delivery applications of chiral mesoporous silica nanoparticles [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4890-4895. |
| [3] | Li Shaoping, Yang Yuqing, Xiao Wenyundeng, Yin Lulu, Liu Libo, Liu Ying, Sun Yifan, Chen Zhiyu. Preparation and characterization of nano-hydroxyapatite/aspirin/polyvinyl alcohol/gelatin/sodium alginate hydrogel scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 3956-3963. |
| [4] | Yu Lanning, Wang Qian, Jin Youshi, Fei Xiaowen, Wang Qingshan. Sealing effect of nano hydroxyapatite on dentinal tubules [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 3964-3970. |
| [5] | Liu Zhuoran, Li Yumei, Liu Junyan, Yin Tong, Jiang Ming, Li Yourui. Role of chitosan and its derivatives in the field of oral antibacterial [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(21): 3361-3367. |
| [6] | Qi Junqiang, Guo Chao, Niu Dongyang, Wang Haotian, Xiao Bing, Xu Guohua. Characteristics and application of bone repair materials of metal ion doped hydroxyapatite [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(21): 3415-3422. |
| [7] | Wang Jianghua, Yin Dongfeng, Teng Yong, Wurikaixi·Aiyiti, Wang Xiaofeng, Mareyanmu·Aini, Jiang Houfeng, Patiguli·Aihemaiti, Wang Jing. Optimization of the preparation process of vancomycin-poly(propylene fumarate)/poly(lactic-co-glycolic acid) microspheres by star point design-response surface method [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(16): 2510-2517. |
| [8] | Diao Xiaojuan, Shi Chaoqun, Li Dong, Wang Shuai, Xiao Chun, Liu Guojun, Qu Tingyu, Yang Weijuan, Jia Xiaopeng. Efficient expansion of natural killer cells from cryopreserved umbilical cord blood by pure cytokine method without animal ingredients [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(10): 1572-1577. |
| [9] | Luo Xiaoling, Zhang Li, Yang Maohua, Xu Jie, Xu Xiaomei. Effect of naringenin on osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1051-1056. |
| [10] | Tan Guozhong, Tu Xinran, Guo Liyang, Zhong Jialin, Zhang Yang, Jiang Qianzhou. Biosafety evaluation of three-dimensional printed gelatin/sodium alginate/58S bioactive glass scaffolds for bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 521-527. |
| [11] | Liu Pei, Zhang Guanying, Yu Quanfeng, Li Zeyu, Han Guangye, Wu Chunlei. Basic fibroblast growth factor combined with poly(lactic acid)/collagen scaffold for urethral defect in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(34): 5468-5474. |
| [12] | Zhang Haonan, Wang Xingran, Li Meimei, Ma Jinjin, Ma Yanxia, Saijilafu. Effect of N-arachidonylethanolamine on axon regeneration of the dorsal root ganglion [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 3999-4003. |
| [13] | Guo Xiaopeng, Liu Yingsong, Shang Hui. Silk fibroin/nano hydroxyapatite composite combined with icariin can promote the proliferation and differentiation of bone marrow mesenchymal stem cells into nucleus pulposus like cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3528-3534. |
| [14] | Lu Haiping, Lang Xuemei, Zhang Cheng, Ju Songli, Zhang Yi, Wang Xin. Application of polycaprolactone modified biological barrier membrane in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3580-3585. |
| [15] | Han Zhi, Wang Zhimiao, Gaxi Sijia, Lu Qingling, Guo Tao. Tissue engineered cartilage constructed by polyurethane composite chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3455-3459. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||