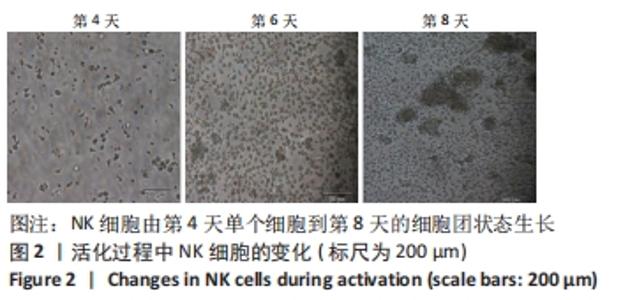
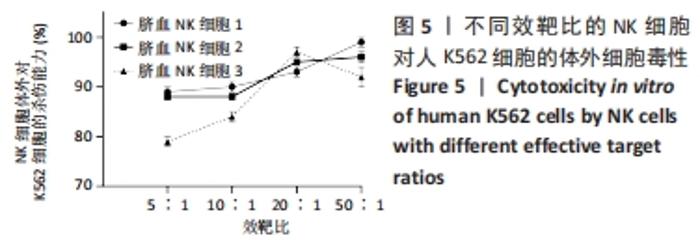
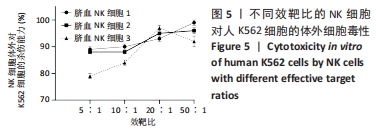
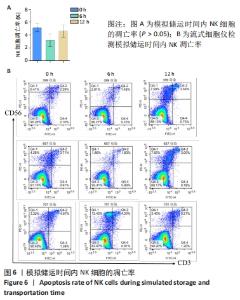
[1] MARTINET L, SMYTH MJ. Balancing natural killer cell activation through paired receptors. Nat Rev Immunol. 2015;15(4):243-254.
[2] HANNA J, MANDELBOIM O. When killers become helpers. Trends Immunol. 2007;28(5):201-206.
[3] KIESSLING R, KLEIN E, WIGZELL H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5(2):112-117.
[4] CHESTER C, FRITSCH K, KOHRT HE. Natural Killer Cell Immunomodulation: Targeting Activating, Inhibitory, and Co-stimulatory Receptor Signaling for Cancer Immunotherapy. Front Immunol. 2015;6:601.
[5] GRANZIN M, WAGNER J, KÖHL U, et al. Shaping of Natural Killer Cell Antitumor Activity by Ex Vivo Cultivation. Front Immunol. 2017;8:458.
[6] MICHEL T, POLI A, CUAPIO A, et al. Human CD56bright NK Cells: An Update. J Immunol. 2016;196(7):2923-2931.
[7] SHERECK E, DAY NS, AWASTHI A, et al. Immunophenotypic, cytotoxic, proteomic and genomic characterization of human cord blood vs. peripheral blood CD56Dim NK cells. Innate Immun. 2019;25(5):294-304.
[8] STOKIC-TRTICA V, DIEFENBACH A, KLOSE CSN. NK Cell Development in Times of Innate Lymphoid Cell Diversity. Front Immunol. 2020;11:813.
[9] MYERS JA, MILLER JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18(2):85-100.
[10] BULLER CW, MATHEW PA, MATHEW SO. Roles of NK Cell Receptors 2B4 (CD244), CS1 (CD319), and LLT1 (CLEC2D) in Cancer. Cancers (Basel). 2020;12(7):1755.
[11] SUTLU T, ALICI E. Natural killer cell-based immunotherapy in cancer: current insights and future prospects. J Intern Med. 2009;266(2):154-181.
[12] MORVAN MG, LANIER LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16(1):7-19.
[13] VIVIER E, TOMASELLO E, BARATIN M, et al. Functions of natural killer cells. Nat Immunol. 2008;9(5):503-510.
[14] PALMER JM, RAJASEKARAN K, THAKAR MS, et al. Clinical relevance of natural killer cells following hematopoietic stem cell transplantation. J Cancer. 2013;4(1):25-35.
[15] CORRADO C, RAIMONDO S, SAIEVA L, et al. Exosome-mediated crosstalk between chronic myelogenous leukemia cells and human bone marrow stromal cells triggers an interleukin 8-dependent survival of leukemia cells. Cancer Lett. 2014;348(1-2):71-76.
[16] ZHU X, YOU Y, LI Q, et al. BCR-ABL1-positive microvesicles transform normal hematopoietic transplants through genomic instability: implications for donor cell leukemia. Leukemia. 2014;28(8):1666-1675.
[17] 王翘楚,郑亮.以肝素和抗 CD16 抗体为基础的扩增人脐血NK 细胞无血清培养体系的建立[J].中国实验血液学杂志,2018,26(2): 552-556.
[18] 汪德海,郭昌龙,周越,等.脐血单核细胞体外高效扩增NK 细胞方法的研究[J].中国输血杂志,2018,31(7):722-726.
[19] CHOI YH, LIM EJ, KIM SW, et al. IL-27 enhances IL-15/IL-18-mediated activation of human natural killer cells. J Immunother Cancer. 2019; 7(1):168.
[20] DAMOISEAUX J. The IL-2 - IL-2 receptor pathway in health and disease: The role of the soluble IL-2 receptor. Clin Immunol. 2020;218:108515.
[21] ZDOLSEK HJ, VEGFORS M, LINDAHL TL, et al. Hydroxyethyl starches and dextran during hip replacement surgery: effects on blood volume and coagulation. Acta Anaesthesiol Scand. 2011;55(6):677-685.
[22] KATAYAMA Y, YANO T, BESSHO A, et al. The effects of a simplified method for cryopreservation and thawing procedures on peripheral blood stem cells. Bone Marrow Transplant. 1997;19(3):283-287.
[23] HAMILTON JR. Albumin-indirect antiglobulin test. Immunohematology. 2019;35(2):63-64.
[24] HU W, WANG G, HUANG D, et al. Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities. Front Immunol. 2019;10:1205.
[25] HODGINS JJ, KHAN ST, PARK MM, et al. Killers 2.0: NK cell therapies at the forefront of cancer control. J Clin Invest. 2019;129(9):3499-3510.
[26] KWEON S, PHAN MT, CHUN S, et al. Expansion of Human NK Cells Using K562 Cells Expressing OX40 Ligand and Short Exposure to IL-21. Front Immunol. 2019;10:879.
[27] THANGARAJ JL, PHAN MT, KWEON S, et al. Expansion of cytotoxic natural killer cells in multiple myeloma patients using K562 cells expressing OX40 ligand and membrane-bound IL-18 and IL-21. Cancer Immunol Immunother. 2022;71(3):613-625.
[28] PHAN MT, LEE SH, KIM SK, et al. Expansion of NK Cells Using Genetically Engineered K562 Feeder Cells. Methods Mol Biol. 2016;1441:167-174.
[29] YANG H, TANG R, LI J, et al. A New Ex Vivo Method for Effective Expansion and Activation of Human Natural Killer Cells for Anti-Tumor Immunotherapy. Cell Biochem Biophys. 2015;73(3):723-729.
[30] MIRANDOLA P, PONTI C, GOBBI G, et al. The response of human natural killer cells to interleukin-2. J Endocrinol Invest. 2004;27(6 Suppl):146-150.
[31] LI Y, LIU B, DING S, et al. Availability of NK cell expansion agent combined with recombinant IL‑2 and IL‑15 stimulation on the expansion and high‑purity of NK cells in patients with immune‑related pancytopenia in vitro. Mol Med Rep. 2019;20(5):4358-4366.
[32] BERGMAN H, LINDQVIST C. Human IL-15 Inhibits NK Cells Specific for Human NK-92 Cells. Anticancer Res. 2021;41(7):3281-3285.
[33] VIDARD L, DUREUIL C, BAUDHUIN J, et al. CD137 (4-1BB) Engagement Fine-Tunes Synergistic IL-15- and IL-21-Driven NK Cell Proliferation. J Immunol. 2019;203(3):676-685.
[34] IKEMIZU S, CHIRIFU M, DAVIS SJ. IL-2 and IL-15 signaling complexes: different but the same. Nat Immunol. 2012;13(12):1141-1142.
[35] WIDOWATI W, JASAPUTRA DK, SUMITRO SB, et al. Effect of interleukins (IL-2, IL-15, IL-18) on receptors activation and cytotoxic activity of natural killer cells in breast cancer cell. Afr Health Sci. 2020;20(2):822-832.
|