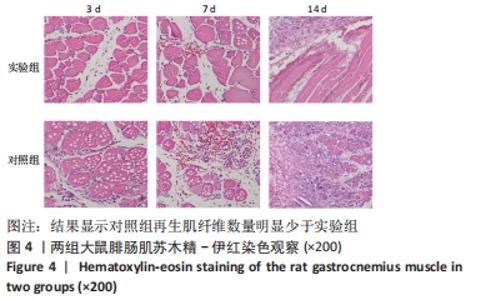
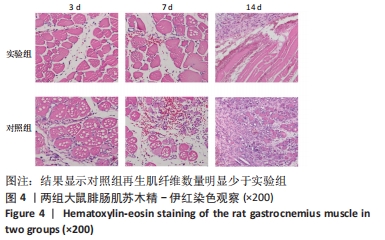
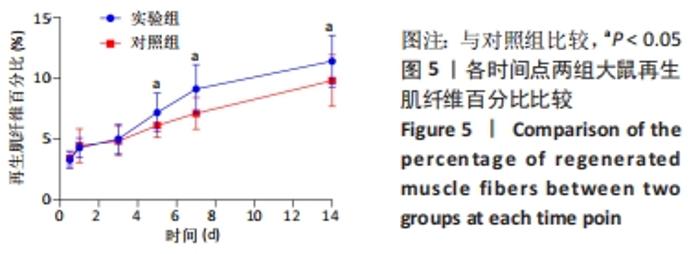
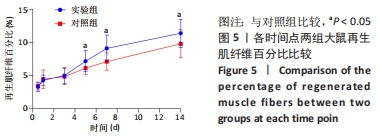
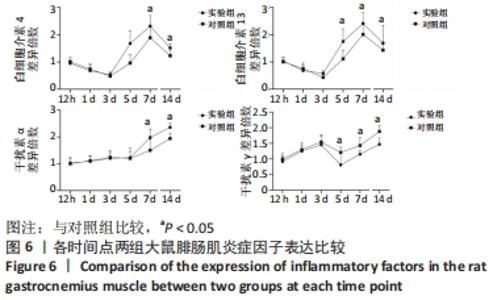
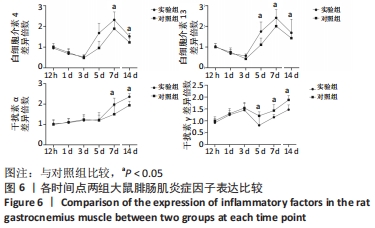
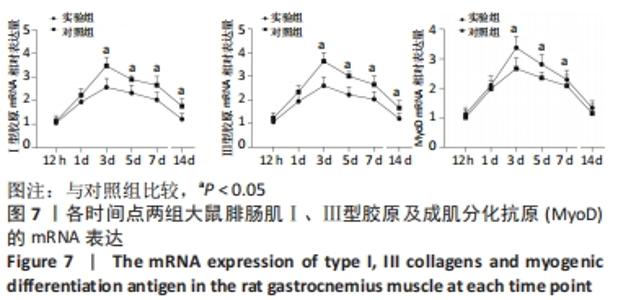
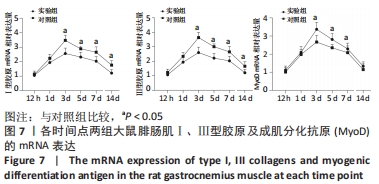
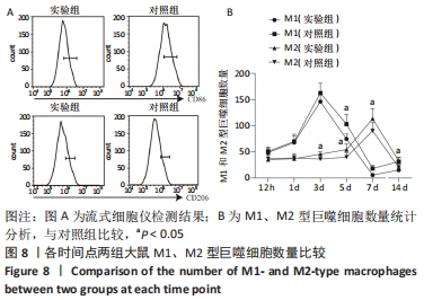
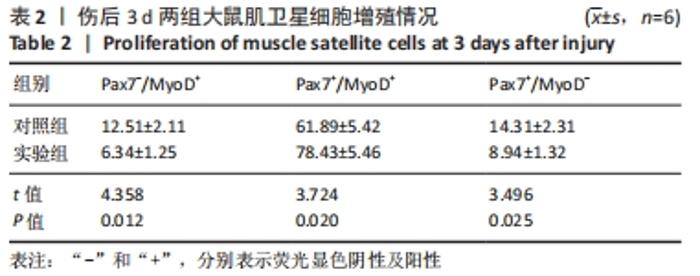
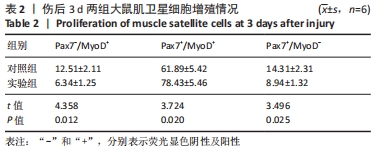
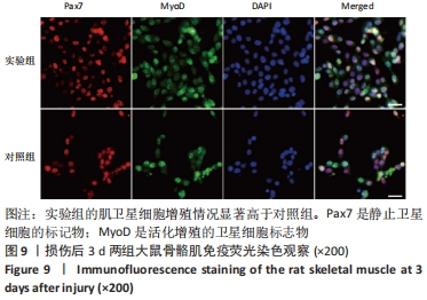
Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (14): 2133-2138.doi: 10.12307/2023.058
Naringenin repairs skeletal muscle injury by regulating polarization of macrophages and proliferation of muscle satellite cells
Xu Mingkui, Xu Riming, Lin Yewu, Luo Yuantai, Zhang Xihui, Zhou Li, Yuan Shiguo
- Second Department of Orthopedics, Chinese Medicine Hospital of Hainan Province, Haikou 570000, Hainan Province, China
-
Received:2022-01-06Accepted:2022-03-17Online:2023-05-18Published:2022-09-30 -
Contact:Zhou Li, Master, Associate chief physician, Second Department of Orthopedics, Chinese Medicine Hospital of Hainan Province, Haikou 570000, Hainan Province, China -
About author:Xu Mingkui, Master, Attending physician, Second Department of Orthopedics, Chinese Medicine Hospital of Hainan Province, Haikou 570000, Hainan Province, China -
Supported by:the Natural Science Foundation of Hainan Province, No. 817340 (to YSG)
CLC Number:
Cite this article
Xu Mingkui, Xu Riming, Lin Yewu, Luo Yuantai, Zhang Xihui, Zhou Li, Yuan Shiguo. Naringenin repairs skeletal muscle injury by regulating polarization of macrophages and proliferation of muscle satellite cells[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(14): 2133-2138.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
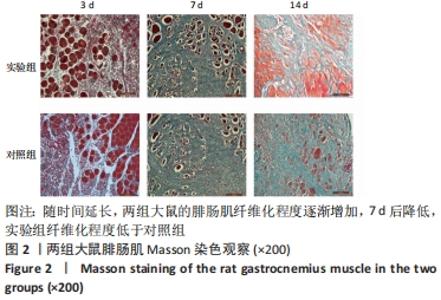
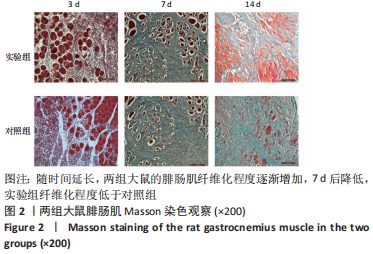
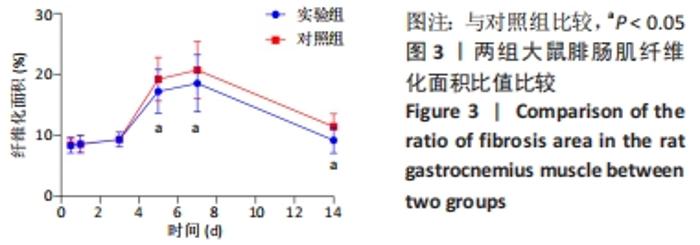
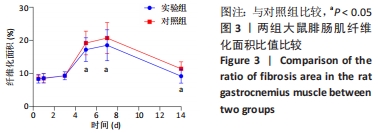
| [1] DEGUCHI H, MORLA S, GRIFFIN JH. Novel blood coagulation molecules: Skeletal muscle myosin and cardiac myosin. J Thromb Haemost. 2021;19(1):7-19. [2] SAHU JK, KUMAR A, PRAKASH K. Randomized controlled trial of electro-acupuncture for autism spectrum disorder. Altern Med Rev. 2010;15(4):302. [3] LI H, CHEN J, CHEN S, et al. Antifibrotic effects of Smad4 small interfering RNAs in injured skeletal muscle after acute contusion. Int J Sports Med. 2011;32(10):735-742. [4] BO LI Z, ZHANG J, WAGNER KR. Inhibition of myostatin reverses muscle fibrosis through apoptosis. J Cell Sci. 2012;125(17):3957-3965. [5] NOGUEIRA JE, AMORIM MR, PINTO AP, et al. Molecular hydrogen downregulates acute exhaustive exercise-induced skeletal muscle damage. Can J Physiol Pharmacol. 2021;99(8):812-820. [6] YE T, ZHONG L, YE X, et al. miR-221-3p and miR-222-3p regulate the SOCS3/STAT3 signaling pathway to downregulate the expression of NIS and reduce radiosensitivity in thyroid cancer. Exp Ther Med. 2021;21(6):652. [7] TUSAVITZ S, KEOONELA S, KALKSTEIN M, et al. Macrophage-derived Wnt signaling increases endothelial permeability during skeletal muscle injury. Inflamm Res. 2020;69(12):1235-1244. [8] SORENSEN JR, KALUHIOKALANI JP, HAFEN PS, et al. An altered response in macrophage phenotype following damage in aged human skeletal muscle: implications for skeletal muscle repair. FASEB J. 2019;33(9):10353-10368. [9] SUN KT, CHEUNG KK, AU SWN, et al.Overexpression of Mechano-Growth Factor Modulates Inflammatory Cytokine Expression and Macrophage Resolution in Skeletal Muscle Injury. Front Physiol. 2018;9:999. [10] LE MOAL E, JUBAN G, BERNARD AS, et al. Macrophage-derived superoxide production and antioxidant response following skeletal muscle injury. Free Radic Biol Med. 2018;120:33-40. [11] WANG X, ZHAO W, RANSOHOFF RM, et al. Infiltrating macrophages are broadly activated at the early stage to support acute skeletal muscle injury repair. J Neuroimmunol. 2018;317:55-66. [12] RYBALKO V, HSIEH PL, MERSCHAM-BANDA M, et al. The Development of Macrophage-Mediated Cell Therapy to Improve Skeletal Muscle Function after Injury. PLoS One. 2015;10(12):e0145550. [13] CÔTÉ CH, BOUCHARD P, VAN ROOIJEN N, et al. Monocyte depletion increases local proliferation of macrophage subsets after skeletal muscle injury. BMC Musculoskelet Disord. 2013;14:359. [14] KRAUSE MP, AL-SAJEE D, D’SOUZA DM, et al. Impaired macrophage and satellite cell infiltration occurs in a muscle-specific fashion following injury in diabetic skeletal muscle. PLoS One. 2013;8(8):e70971. [15] 程建红,洪莉,洪莎莎,等.巨噬细胞在骨骼肌损伤再生中的研究进展[J].医学综述,2021,27(16):3125-3129. [16] 贺舟,常青,唐成林,等.大鼠骨骼肌急性损伤后早期运动训练和按摩对肌卫星细胞增殖相关因子的影响[J].中国康复理论与实践,2020,26(1):49-54. [17] 胡久婷,朱道立.慢肌卫星细胞移植修复骨骼肌的钝挫损伤[J].中国组织工程研究与临床康复,2010,14(23):4271-4274. [18] 谢春燕,谢刚,季语竹.柚皮素通过miR-22抑制NLRP3炎症小体并减轻溃疡性结肠炎大鼠模型肠屏障损伤[J].中国病理生理杂志,2021,37(9):1573-1581. [19] 郭诗哲,孙亚英,刘少华, 等.柚皮素抑制小鼠骨骼肌急性钝挫伤后纤维化[J].中国运动医学杂志,2017,36(3):201-206. [20] CHAZAUD B. Inflammation and Skeletal Muscle Regeneration: Leave It to the Macrophages!. Trends Immunol. 2020;41(6):481-492. [21] TIAN ZL, JIANG SK, ZHANG M, et al. Detection of satellite cells during skeletal muscle wound healing in rats: time-dependent expressions of Pax7 and MyoD in relation to wound age. Int J Legal Med. 2016;130(1):163-172. [22] SATO T, HIGASHIOKA K, SAKURAI H, et al. Core Transcription Factors Promote Induction of PAX3-Positive Skeletal Muscle Stem Cells. Stem Cell Reports. 2019; 13(2):352-365. [23] ALMEIDA CF, FERNANDES SA, RIBEIRO JUNIOR AF, et al. Muscle Satellite Cells: Exploring the Basic Biology to Rule Them. Stem Cells Int. 2016;16(3):1078686. [24] FUJIMAKI S, SEKO D, KITAJIMA Y, et al. Notch1 and Notch2 Coordinately Regulate Stem Cell Function in the Quiescent and Activated States of Muscle Satellite Cells.Stem Cells. 2018;36(2):278-285. [25] HUŠÁKOVÁ M, BAY-JENSEN AC, FOREJTOVÁ Š, et al. Metabolites of type I, II, III, and IV collagen may serve as markers of disease activity in axial spondyloarthritis. Sci Rep. 2019;9(1):11218. [26] RHO SW, CHOI GS, KO EJ, et al. Molecular changes in remote tissues induced by electro-acupuncture stimulation at acupoint ST36. Mol Cells. 2008,25(2):178-183. [27] ARANGO DUQUE G, DESCOTEAUX A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;7(5):491. [28] DU H, SHIH CH, WOSCZYNA MN, et al. Macrophage-released ADAMTS1 promotes muscle stem cell activation. Nat Commun. 2017;8(1):669. [29] SU Y, YU Y, LIU C, et al. Fate decision of satellite cell differentiation and self-renewal by miR-31-IL34 axis. Cell Death Differ. 2020;27(3):949-965. [30] MCLENNAN IS. Degenerating and regenerating skeletal muscles contain several subpopulations of macrophages with distinct spatial and temporal distributions. J Anat. 1996;188( Pt 1):17-28. [31] WANG X, SATHE AA, SMITH GR, et al. Heterogeneous origins and functions of mouse skeletal muscle-resident macrophages. Proc Natl Acad Sci U S A. 2020; 117(34):20729-20740. [32] CARLETON MM, SEFTON MV. Injectable and degradable methacrylic acid hydrogel alters macrophage response in skeletal muscle. Biomaterials. 2020;223:119477. [33] SONG S, AN J, LI Y, et al. Electroacupuncture at ST-36 ameliorates DSS-induced acute colitis via regulating macrophage polarization induced by suppressing NLRP3/IL-1beta and promoting Nrf2/HO-1. Mol Immunol. 2019;106:143-152. [34] MCAVOY M, TSOSIE JK, VYAS KN, et al. Flexible Multielectrode Array for Skeletal Muscle Conditioning, Acetylcholine Receptor Stabilization and Epimysial Recording After Critical Peripheral Nerve Injury. Theranostics. 2019;9(23):7099-7107. [35] CUI CY, DRISCOLL RK, PIAO Y, et al. Skewed macrophage polarization in aging skeletal muscle. Aging Cell. 2019;18(6):e13032. [36] LI T, GARCIA-GOMEZ A, MORANTE-PALACIOS O, et al. SIRT1/2 orchestrate acquisition of DNA methylation and loss of histone H3 activating marks to prevent premature activation of inflammatory genes in macrophages. Nucleic Acids Res. 2020;48(2):665-681. [37] HE L, ZHAO X, HE L. LINC01140 Alleviates the Oxidized Low-Density Lipoprotein-Induced Inflammatory Response in Macrophages via Suppressing miR-23b. Inflammation. 2020;43(1):66-73. [38] MCCLAIN CALDWELL I, HOGDEN C, NEMETH K, et al. Bone Marrow-Derived Mesenchymal Stromal Cells (MSCs) Modulate the Inflammatory Character of Alveolar Macrophages from Sarcoidosis Patients. J Clin Med. 2020;9(1):278. |
| [1] | Guo Shuhui, Yang Ye, Jiang Yangyang, Xu Jianwen. Screening and validation of neurogenic bladder miRNA-mRNA regulatory network [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-8. |
| [2] | He Xi, Wan Yu, Tang Yuting, Yang Anning, Wu Kai, Jiao Yun, Bai Zhigang, Jiang Yideng, Shen Jiangyong. Erastin inhibits proliferation of hypertrophic scar fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-. |
| [3] | Zhong Yizheng, Huang Peizhen, Cai Qunbin, Zheng Liqin, He Xingpeng, Dong Hang. Microstructural indexes that determine the trabecular bone maximum stress of micro-finite element models [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1313-1318. |
| [4] | Cao Sheng, Kong Lingwei, Xu Kun, Sun Zhijie. Correlation of cervical sagittal force line parameters with degenerative segment and Pfirrmann classification in patients with cervical intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1319-1324. |
| [5] | Ke Yuqi, Chen Changjian, Wu Hao, Zheng Lianjie. Comparison of 12-month follow-up results of primary total hip arthroplasty between modified direct anterior approach and direct anterior approach [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1377-1382. |
| [6] | Zhang Lichuang, Gao Huali, Wang Jingchao, Lin Huijun, Wu Chonggui, Ma Yinghui, Huang Yunfei, Fang Xue, Zhai Weitao. Effect of tendon manipulation with equal emphasis on muscles and bones on accelerating the functional rehabilitation of quadriceps femoris after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1383-1389. |
| [7] | Du Xueting, Zhang Xiaodong, Chen Yanjun, Wang Mei, Chen Wubiao, Huang Wenhua. Application of compressed sensing technology in two-dimensional magnetic resonance imaging of the ankle joint [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1396-1402. |
| [8] | You Zhengqiu, Zhang Zhongzu, Wang Qunbo. Early symptomatic intervertebral disc pseudocysts after discectomy detected on MRI [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1403-1409. |
| [9] | Li Chao, Zhang Peipei, Xu Mengting, Li Linlin, Ding Jiangtao, Liu Xihua, Bi Hongyan. Respiratory training improves morphological changes of the multifidus muscle in patients with chronic nonspecific lower back pain assessed by musculoskeletal ultrasound [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1417-1421. |
| [10] | He Yinhao, Li Xiaosheng, Chen Hongwen, Chen Tiezhu. 3D printed porous tantalum metal in the treatment of developmental dysplasia of the hip: current status and application prospect [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1455-1461. |
| [11] | Jiang Xiaocheng, Shi Lu, Wang Yinbin, Li Qiujiang, Xi Chuangzhen, Ma Zefeng, Cai Lijun. Systematical evaluation of bone fusion rate after interbody fusion in patients with osteoporosis and lumbar degenerative disease treated with teriparatide [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1427-1433. |
| [12] | Wu Dongzhe, Gao Xiaolin, Li Chuangtao, Wang Hao. Constructing the prediction model of maximal oxygen uptake by back-propagation neural network based on the cardiorespiratory optimal point [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1224-1231. |
| [13] | Yang Jiujie, Li Zhi, Wang Shujie, Tian Ye, Zhao Wei. Intraoperative neurophysiological monitoring of functional changes following durotomy with decompression for acute spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1232-1236. |
| [14] | Nie Chenchen, Su Kaiqi, Gao Jing, Fan Yongfu, Ruan Xiaodi, Yuan Jie, Duan Zhaoyuan, Feng Xiaodong. The regulatory role of circular RNAs in cerebral ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1286-1291. |
| [15] | Gao Yu, Han Jiahui, Ge Xin. Immunoinflammatory microenvironment after spinal cord ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1300-1305. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||