Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (14): 2176-2181.doi: 10.12307/2023.059
Previous Articles Next Articles
Notch2 contributes to the development of aortic and pulmonary valves in mouse embryonic heart
Wang Jiajia, Xie Jianshan, Li Hairong, Jing Yixin, Yang Yanping
- Department of Histology and Embryology, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
-
Received:2022-02-14Accepted:2022-03-11Online:2023-05-18Published:2022-09-30 -
Contact:Yang Yanping, MD, Professor, Department of Histology and Embryology, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
About author:Wang Jiajia, Master candidate, Department of Histology and Embryology, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
Supported by:the National Natural Science Foundation of China, No. 31200899 (to YYP)
CLC Number:
Cite this article
Wang Jiajia, Xie Jianshan, Li Hairong, Jing Yixin, Yang Yanping. Notch2 contributes to the development of aortic and pulmonary valves in mouse embryonic heart[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(14): 2176-2181.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

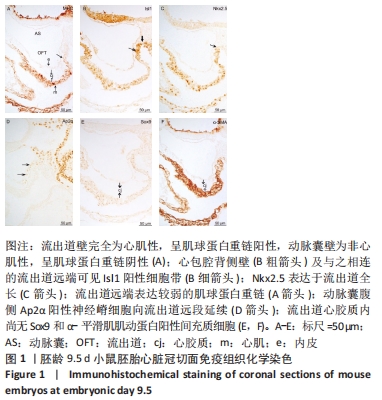
2.1 小鼠胚胎流出道心内膜垫与插入垫的形成 胚龄9.5 d小鼠胚胎, 流出道呈单管状,其管壁由外向内依次可见心肌膜、无细胞的心胶质及内皮,见图1A。Isl1为第二生心区前体细胞的特异性标记物,表达见于心包腔背侧壁的脏壁中胚层,并延续至与之相连的流出道远端壁,见图1B。Nkx2.5用于显示第二生心区前体细胞、早期分化以及分化完成的心肌细胞,由流出道远端至近端,均可见Nkx2.5阳性细胞,见图1C。心肌细胞标记物MHC在流出道心肌膜远端呈弱阳性表达,向流出道近端延伸,MHC表达增强,见图1A。 Isl1、Nkx2.5与MHC在心包腔背侧壁与流出道远端壁的表达模式提示第二生心区前体细胞向心肌细胞分化;MHC、Nkx2.5在流出道的表达模式则说明此期流出道为心肌性流出道,见图1A,C。此期动脉囊位于咽腹侧,管壁由内皮构成,其近端与心包内的流出道相连,见图1A。动脉囊与弓动脉周围可见正在迁移的神经嵴细胞的标记物Ap2α的表达。动脉囊腹侧的Ap2α阳性细胞延续至流出道远段的心胶质内,见图1D,提示神经嵴细胞沿着弓动脉、动脉囊壁迁移进入流出道。Sox9、α-平滑肌肌动蛋白可用于标记间充质细胞。连续切片观察,流出道心胶质内尚未出现Sox9或α-平滑肌肌动蛋白阳性间充质细胞,见图1E,F。"
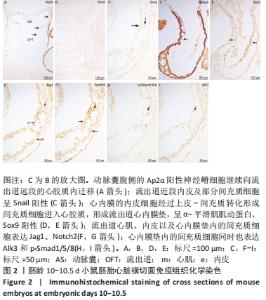
胚龄10-10.5 d,动脉囊腹侧的Ap2α阳性神经嵴细胞继续延伸至流出道远段的心胶质内,见图2A。流出道内皮及其邻近的部分间充质细胞开始表达上皮-间充质转化特异性标记物Snail,尤以流出道近段较明显,见图2B,C,说明心内膜的内皮细胞经过上皮-间充质转化形成心胶质内的间充质细胞。神经嵴细胞的迁入与内皮细胞的上皮-间充质转化使流出道心胶质内间充质细胞明显可见,并表达α-平滑肌肌动蛋白、Sox9,见图2D,E,说明流出道心内膜垫形成。流出道心肌、内皮以及心内膜垫内的间充质细胞同时表达Jag1、Notch2,见图2F,G,心内膜垫内的间充质细胞也表达Alk3和p-Smad1/5/8,见图2H,I。 "
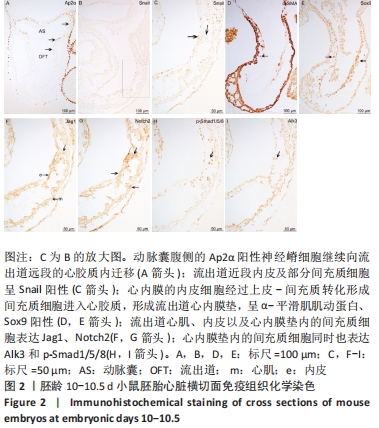

胚龄11 d,咽腹侧的Isl1阳性细胞急剧增加,形成锥形区,见图3A,部分细胞表达Nkx2.5,见图3B。此期动脉囊由心包背侧突入心包腔,MHC染色呈阴性,因此形成流出道非心肌部,其远端壁仍与心包腔背侧壁相连,见图3C。横切面和冠状切面显示上述锥形区域尖端Isl1或Nkx2.5阳性细胞经左、右弓动脉之间延续入流出道非心肌部的内皮与外层上皮之间,在局部聚集形成阳性细胞团,即插入垫,见图3A,B,D,E。相邻切片染色显示此处流出道壁的外表面MHC仅局部表达,见图3F,说明插入垫出现于流出道非心肌部与心肌部交界处。连续切片观察肺动脉侧插入垫内Nkx2.5阳性细胞多于主动脉侧插入垫,见图3G,说明前者的形成早于后者;三维重建结果显示肺动脉侧插入垫定位高于主动脉侧插入垫,见图3H,I,J。比较两种心内膜垫,可见插入垫和流出道心内膜垫几乎出现于同一水平,但插入垫的长度小于流出道心内膜垫的长度,见图3H;另一方面,流出道心内膜垫内可见α-平滑肌肌动蛋白、Sox9、Jag1、Notch2、p-Smad1/5/8与Alk3的表达,见图3K-P,但不再表达Snail,见图3Q。而上述包括Snail在内的7种蛋白在插入垫内大多呈阴性,见图3K-N,P,仅可见p-Smad1/5/8阳性细胞,见图3O,说明插入垫形成时并未发生上皮-间充质转化。至胚龄11.5 d,两侧插入垫大小对称,仍富含Isl1阳性细胞,见图3R,但Nkx2.5表达减少,见图3S,可观察到少量Sox9阳性细胞,见图3T。 "
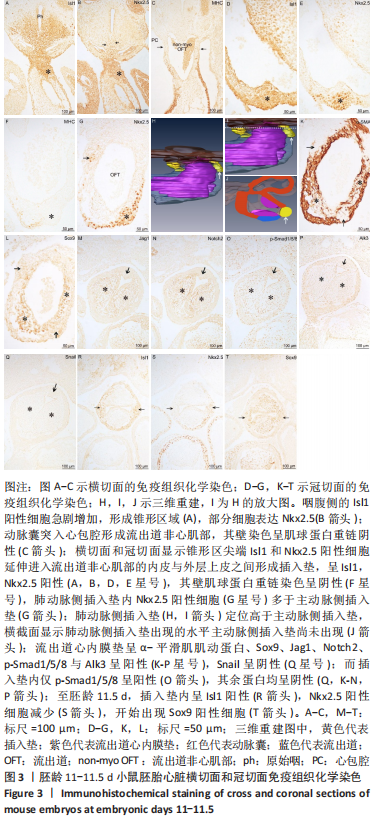

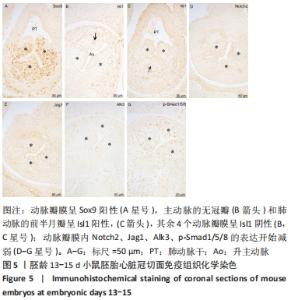
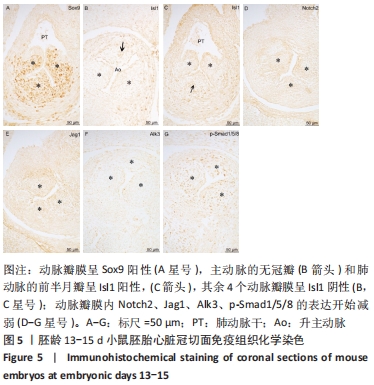
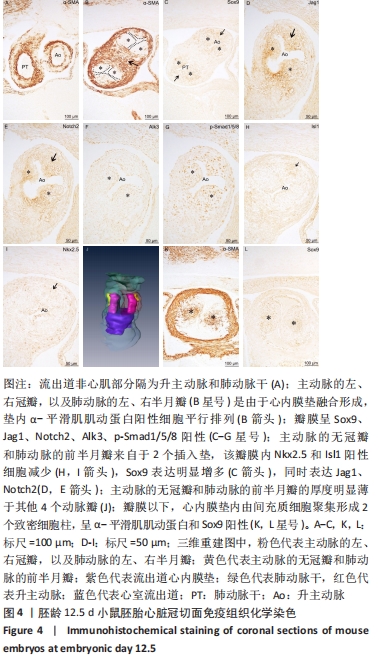
2.2 小鼠胚胎流出道分隔与主、肺动脉瓣膜形成 胚龄12.5 d,流出道的非心肌部已经分隔为升主动脉和肺动脉干,见图4A。在两动脉根部与流出道心肌部交界处,动脉瓣膜原基已形成,其中主动脉的左、右冠瓣,以及肺动脉的左、右半月瓣是由于心内膜垫融合形成,见图4B。瓣膜内富含Sox9阳性间充质细胞,见图4C,也可观察到Jag1、Notch2、Alk3、p-Smad1/5/8的明显表达,见图4D-G。而主动脉的无冠瓣和肺动脉的前半月瓣来自于2个插入垫,与胚龄11.5 d相比,该瓣膜内Isl1阳性细胞和Nkx2.5阳性细胞急剧减少,见图4H,I,Sox9表达明显上调,见图4C,同时开始表达Jag1和Notch2,见图4D,E。在此期,连续切片观察和三维重建结果显示主动脉的无冠瓣和肺动脉的前半月瓣的厚度明显薄于其他4个动脉瓣,见图4J。瓣膜以下,流出道心内膜垫彼此相贴,但尚未融合,心内膜垫内由间充质细胞聚集形成2个致密细胞柱,所含细胞大多呈α-平滑肌肌动蛋白阳性,见图4K,也表达Sox9,见图4L。在瓣膜水平,两侧致密细胞柱在中线融合,α-平滑肌肌动蛋白阳性细胞平行排列,见图4B。"
| [1] BRAVO-JAIMES K, PRAKASH SK. Genetics in bicuspid aortic valve disease: Where are we? Prog Cardiovasc Dis. 2020;63(4):398-406. [2] YANG YP, LI HR, CAO XM, et al. Septation of the Intrapericardial Arterial Trunks in the Early Human Embryonic Heart. Chin Med J (Engl). 2018; 131(12):1457-1464. [3] YAMAGISHI T, NAREMATSU M, NAKAJIMA Y. Msx1 upregulates p27 expression to control cellular proliferation during valvuloseptal endocardial cushion formation in the chick embryonic heart. Anat Rec (Hoboken). 2021;304(8):1732-1744. [4] CORTES C, FRANCOU A, DE BONO C, et al. Epithelial Properties of the Second Heart Field. Circ Res. 2018;122(1):142-154. [5] SCHUSSLER O, GHARIBEH L, MOOTOOSAMY P, et al. Cardiac Neural Crest Cells: Their Rhombomeric Specification, Migration, and Association with Heart and Great Vessel Anomalies. Cell Mol Neurobiol. 2021;41(3):403-429. [6] MIFFLIN JJ, DUPUIS LE, ALCALA NE, et al. Intercalated cushion cells within the cardiac outflow tract are derived from the myocardial troponin T type 2 (Tnnt2) Cre lineage. Dev Dyn. 2018;247(8):1005-1017. [7] ODELIN G, FAURE E, COULPIER F, et al. Krox20 defines a subpopulation of cardiac neural crest cells contributing to arterial valves and bicuspid aortic valve. Development. 2018;145(1):dev151944. [8] KURATANI SC, KIRBY ML. Initial migration and distribution of the cardiac neural crest in the avian embryo: an introduction to the concept of the circumpharyngeal crest. Am J Anat. 1991;191(3):215-227. [9] ANDERSON R, CHAUDHRY B, MOHUN T, et al. Normal and abnormal development of the intrapericardial arterial trunks in humans and mice. Cardiovasc Res. 2012;95(1):108-115. [10] YANG YP, LI HR, JING Y. Septation and shortening of outflow tract in embryonic mouse heart involve changes in cardiomyocyte phenotype and alpha-SMA positive cells in the endocardium. Chin Med J (Engl). 2004;117(8):1240-1245. [11] RAMIREZ A, ASTROF S. Visualization and Analysis of Pharyngeal Arch Arteries using Whole-mount Immunohistochemistry and 3D Reconstruction. J Vis Exp. 2020;(157):10.3791/60797. [12] ELEY L, ALQAHTANI AM, MACGROGAN D, et al. A novel source of arterial valve cells linked to bicuspid aortic valve without raphe in mice. Elife. 2018;7:e34110. [13] ANDERSON RH, WEBB S, BROWN NA, et al. Development of the heart: (3) formation of the ventricular outflow tracts, arterial valves, and intrapericardial arterial trunks. Heart. 2003;89(9):1110-1118. [14] SOTO-NAVARRETE MT, LÓPEZ-UNZU MÁ, DURÁN AC, et al. Embryonic development of bicuspid aortic valves. Prog Cardiovasc Dis. 2020; 63(4):407-418. [15] FRANCOU A, SAINT-MICHEL E, MESBAH K, et al. Second heart field cardiac progenitor cells in the early mouse embryo. Biochim Biophys Acta. 2013;1833(4):795-798. [16] DE BONO C, THELLIER C, BERTRAND N, et al. T-box genes and retinoic acid signaling regulate the segregation of arterial and venous pole progenitor cells in the murine second heart field. Hum Mol Genet. 2018;27(21):3747-3760. [17] MILOS NC, NORDSTROM DB, ONGARO I, et al. Variations in structure of the outflow tract of the human embryonic heart: A new hypothesis for generating bicuspid aortic semilunar valves. Ann Anat. 2017;211:88-103. [18] MACGROGAN D, LUNA-ZURITA L, DE LA POMPA JL. Notch signaling in cardiac valve development and disease. Birth Defects Res A Clin Mol Teratol. 2011;91(6):449-459. [19] HARRISON OJ, VISAN AC, MOORJANI N, et al. Defective NOTCH signaling drives increased vascular smooth muscle cell apoptosis and contractile differentiation in bicuspid aortic valve aortopathy: A review of the evidence and future directions. Trends Cardiovasc Med. 2019;29(2):61-68. [20] GARG V, MUTH AN, RANSOM JF, et al. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437(7056):270-274. [21] KAUFMAN MH. The Atlas of Mouse Developmet. US: academeic press. 1992:5-8. [22] VARADKAR P, KRAMAN M, DESPRES D, et al. Notch2 is required for the proliferation of cardiac neural crest-derived smooth muscle cells. Dev Dyn. 2008;237(4):1144-1152. [23] HIGH FA, JAIN R, STOLLER JZ, et al. Murine Jagged1/Notch signaling in the second heart field orchestrates Fgf8 expression and tissue-tissue interactions during outflow tract development. J Clin Invest. 2009; 119(7):1986-1996. [24] LIN CJ, LIN CY, CHEN CH, et al. Partitioning the heart: mechanisms of cardiac septation and valve development. Development. 2012;139(18): 3277-3299. [25] WANG W, HU YF, PANG M, et al. BMP and Notch Signaling Pathways differentially regulate Cardiomyocyte Proliferation during Ventricle Regeneration. Int J Biol Sci. 2021;17(9):2157-2166. [26] ZHAO HJ, KLAUSEN C, LI Y, et al. Bone morphogenetic protein 2 promotes human trophoblast cell invasion by upregulating N-cadherin via non-canonical SMAD2/3 signaling. Cell Death Dis. 2018;9(2):174. [27] THIERY JP, SLEEMAN JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131-142. [28] 杨艳萍, 赵瑞庆, 李海荣, 等. 小鼠胚胎心脏流出道嵴的发育 [J]. 解剖学报,2010,41(1):80-86. [29] MACGROGAN D, D’AMATO G, TRAVISANO S, et al. Sequential Ligand-Dependent Notch Signaling Activation Regulates Valve Primordium Formation and Morphogenesis. Circ Res. 2016;118(10):1480-1497. [30] JAIN R, ENGLEKA K, RENTSCHLER S, et al. Cardiac neural crest orchestrates remodeling and functional maturation of mouse semilunar valves. J Clin Invest. 2011;121(1):422-430. |
| [1] | Song Jian, Zhao Lei, Liu Aishi. Construction and application of myocardial ischemia model in miniature pigs [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 772-778. |
| [2] | Wang Shuo, Liu Wenying, Lü Chaofan, Li Jiacong, Geng Yi, Zhao Yungang. Cardioprotective effect of 3-nitro-N-methyl salicylamide on the isolated rat heart under cold ischemia preservation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1194-1201. |
| [3] | Sun Ying, Xiang Guangda, Xu Xiaoli. Effects of myeloid-derived growth factor on ventricular remodeling in aging mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(31): 5020-5025. |
| [4] | Zhao Lin, Fan Chenxing, Li Kun. Mechanism underlying tanshinone IIA effect on survival and homing ability of myocardial precursor cells under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(30): 4852-4856. |
| [5] | Tian Min, Liu Yuting, Xie Like, Cao Yang, Lyu Hongbin. Protective effects of tertiary butylhydroquinone on various organs in type 2 diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(29): 4616-4623. |
| [6] | Duan Zhengwei, Liu Yunfei. Conventional versus marginal donor heart transplantation in patients with end-stage heart disease [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(26): 4211-4215. |
| [7] | Zhou Shicheng, Han Hongguang, Ji Fang, Xu Liying, Zhang Xiaohui, Sun Chang. Effect and histocompatibility of expended polytetrafluoroethylene in modified Blalock-Taussig shunt [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(21): 3394-3400. |
| [8] | Zhang Haiyang, Bi Shengli, Feng Jingru, Li Fan, Wang Jing, Zhao Na, Li Xinjun. Effects of silent information regulator 1 on chronic heart failure in a postmenopausal mouse model and its mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(20): 3184-3189. |
| [9] | Zhu Zheng, Fu Changxi, Ma Wenchao, Ma Gang, Peng peng. Regulatory mechanism of aerobic exercise on cardiac remodeling in spontaneously hypertensive rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(14): 2231-2237. |
| [10] | Zhang Yang, Zhao Qiaoyan, Zhai Jiwei, Li Jing . Changes in serum inflammatory factors and hemodynamics of biological mitral valve replacement for elderly patients with cardiac valve disease [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1561-1565. |
| [11] | Zhang Wenwen, Jin Songfeng, Zhao Guoliang, Gong Lihong. Mechanism by which Wenban Decoction reduces homocysteine-induced apoptosis of myocardial microvascular endothelial cells in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 723-728. |
| [12] | Zhang Qunhui, Li Yimei, Zhang Dejun. Hypoxia-inducible factor and coronary heart disease: antagonism and protection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(35): 5729-5734. |
| [13] | Hui Xiaoshan, Zhang Jinsheng, He Qingyong, Wang Shiqi, Yang Shuai, Zhang Hui, Wang Jie. Cardiomyocyte direct reprogramming: a scientometric and visualization analysis based on the Web of Science [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(32): 5123-5131. |
| [14] | Lai Shuaiwei, Zhang Shasha, Liu Xiaoyun, Haniya Mazhar, Amber Naz, He Lin, Zhu Hongxin. Ultraviolet resistance-associated gene (Uvrag) deficiency promotes cellular senescence in the heart [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(14): 2241-2246. |
| [15] | Mao Xin, Yu Limei, Wang Feng. Important role of mesenchymal stem cells in immune tolerance induction in heart transplantation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(13): 2070-2078. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||