Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (10): 1484-1491.doi: 10.12307/2023.268
Previous Articles Next Articles
Effect of adipose-derived stem cells stimulated by direct current electric field on refractory wound healing in diabetic rats
Zhang Rui, Liu Lan, Xie Defu, Lin Yingxi, Ren Hongjing, Yan Hong
- Department of Plastic and Burn Surgery, Affiliated Hospital of Southwest Medical University, National Key Clinical Construction Specialty, Wound Repair and Regeneration Laboratory, Luzhou 646000, Sichuan Province, China
-
Received:2022-04-07Accepted:2022-05-13Online:2023-04-08Published:2022-09-07 -
Contact:Yan Hong, MD, Chief physician, Department of Plastic and Burn Surgery, Affiliated Hospital of Southwest Medical University, National Key Clinical Construction Specialty, Wound Repair and Regeneration Laboratory, Luzhou 646000, Sichuan Province, China -
About author:Zhang Rui, Master candidate, Department of Plastic and Burn Surgery, Affiliated Hospital of Southwest Medical University, National Key Clinical Construction Specialty, Wound Repair and Regeneration Laboratory, Luzhou 646000, Sichuan Province, China Liu Lan, Master, Physician, Department of Plastic and Burn Surgery, Affiliated Hospital of Southwest Medical University, National Key Clinical Construction Specialty, Wound Repair and Regeneration Laboratory, Luzhou 646000, Sichuan Province, China Zhang Rui and Liu Lan contributed equally to this article. -
Supported by:the Strategy and Cooperation Project of Southwest Medical University-Luzhou People’s Government, No. 2019LZXNYDZ08 (to YH); School-Level Scientific Research Project of Southwest Medical University, No. 2021ZKQN074 (to LL)
CLC Number:
Cite this article
Zhang Rui, Liu Lan, Xie Defu, Lin Yingxi, Ren Hongjing, Yan Hong. Effect of adipose-derived stem cells stimulated by direct current electric field on refractory wound healing in diabetic rats[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(10): 1484-1491.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
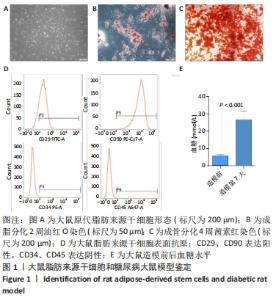
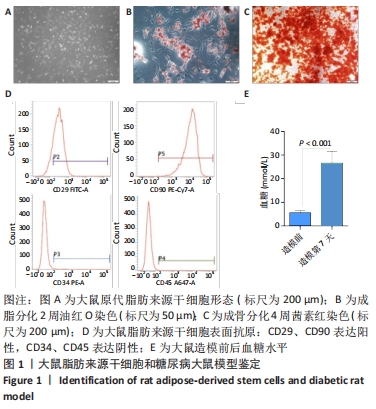
2.1 大鼠脂肪来源干细胞形态观察及鉴定结果 大鼠脂肪来源干细胞提取后接种在培养皿中,24 h后倒置显微镜观察显示,细胞形态大多呈梭形,少数呈三角形或多边形,见图1A。第3代大鼠脂肪来源干细胞成脂诱导过程中,细胞内逐渐出现形态大小不一的脂滴,诱导2周后油红O染色呈阳性;成骨诱导4周的过程中,大鼠脂肪来源干细胞的形态逐渐由梭形变为多边形,可观察到细胞间有钙结节形成,茜素红染色呈阳性,见图1B,C。流式细胞术结果表明大鼠脂肪来源干细胞表面抗原CD29、CD90阳性表达率分别为84.51%,99.95%,CD34、CD45的阳性表达率分别为0.52%,0.06%,见图1D。 2.2 糖尿病大鼠模型评估结果 注射链脲佐菌素后第3,5,7天大鼠随机血糖值均大于正常值16.7 mmol/L,大鼠第7天随机血糖(26.66±4.91) mmol/L较造模前(5.68±0.18) mmol/L明显增加(P < 0.001),说明糖尿病大鼠模型是合格的,见图1E。"
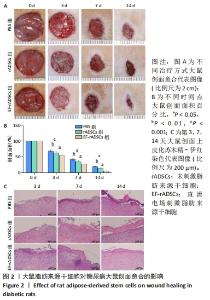
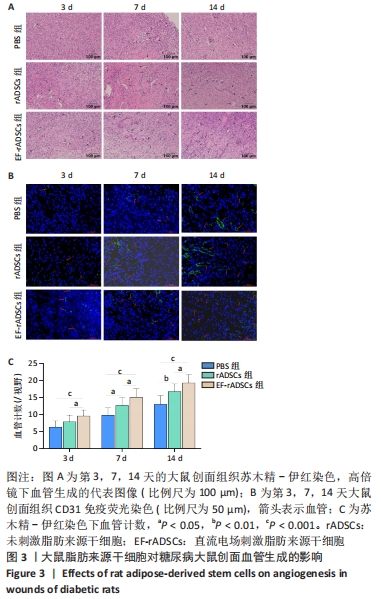
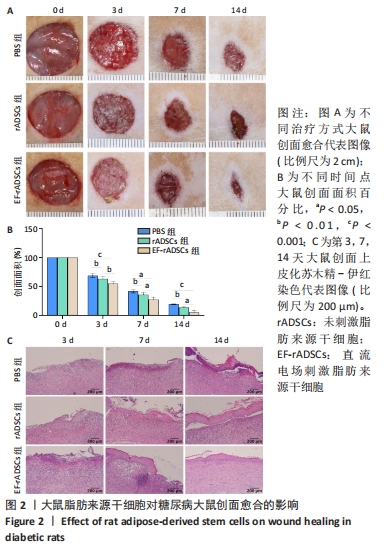
2.3 直流电场作用后的大鼠脂肪来源干细胞对糖尿病大鼠难愈性创面愈合的影响 2.3.1 大鼠脂肪来源干细胞促进糖尿病大鼠创面愈合 测量大鼠治疗前(0 d)和治疗后3,7,14 d创面愈合率,结果表明:PBS组各时间点创面愈合率均明显低于未刺激脂肪来源干细胞组和直流电场刺激脂肪来源干细胞组(P < 0.05),与未刺激脂肪来源干细胞组相比,直流电场刺激脂肪来源干细胞组的创面愈合速度更快(P < 0.05)。第14天,直流电场刺激脂肪来源干细胞组创面已经基本愈合,愈合率达到95%,未刺激脂肪来源干细胞组愈合率为87%,PBS组为81%,见图2A,B,表明直流电场作用后的脂肪来源干细胞可促进糖尿病大鼠创面愈合,其促进糖尿病大鼠创面愈合的效率显著高于未刺激组。 2.3.2 大鼠脂肪来源干细胞促进创面上皮化 大鼠皮肤全层缺损后表现为表皮、真皮层结构明显缺失。术后第3天,各组大鼠创面均未见明显上皮化,可见大量炎症细胞;治疗第7天,直流电场刺激脂肪来源干细胞组表皮及真皮增生更加明显;治疗第14天,各组基本形成表皮及真皮结构,其中直流电场刺激脂肪来源干细胞组的表皮较其他组更完整,而PBS组表皮则不连续,未刺激脂肪来源干细胞组则介于二者之间,各组炎性细胞均明显减少,见图2C。"

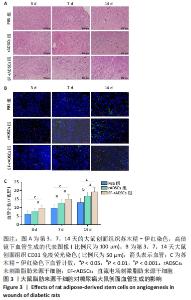
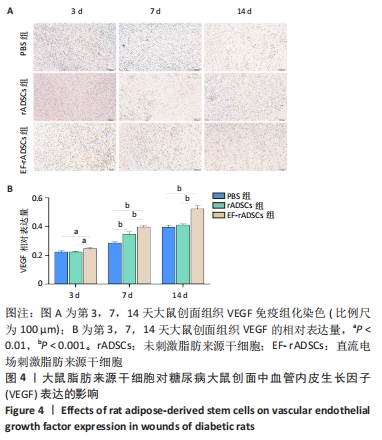
2.3.4 大鼠脂肪来源干细胞促进血管内皮生长因子的生成 血管生成与血管内皮生长因子等细胞因子关系密切,为此通过免疫组化方法对不同组间创面组织血管内皮生长因子的表达进行图像软件分析。数据显示:随着创面愈合的进展各组血管内皮生长因子的表达逐渐增加,治疗第3天,直流电场刺激脂肪来源干细胞组中可见较少血管内皮生长因子表达,但多于未刺激脂肪来源干细胞组和PBS组(P < 0.05);第7天时各组大鼠创面血管内皮生长因子表达明显增加,与未刺激脂肪来源干细胞组和PBS组相比,直流电场刺激脂肪来源干细胞组血管内皮生长因子表达增加明显(P < 0.05),未刺激脂肪来源干细胞组和PBS组间也存在明显差异(P < 0.05);第14天时创面组织血管内皮生长因子表达丰富,其中直流电场刺激脂肪来源干细胞组表达最多的血管内皮生长因子(P < 0.05),其余两组无明显差异,见图4。"
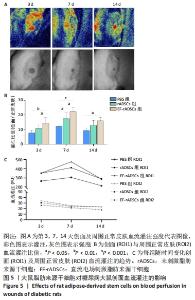
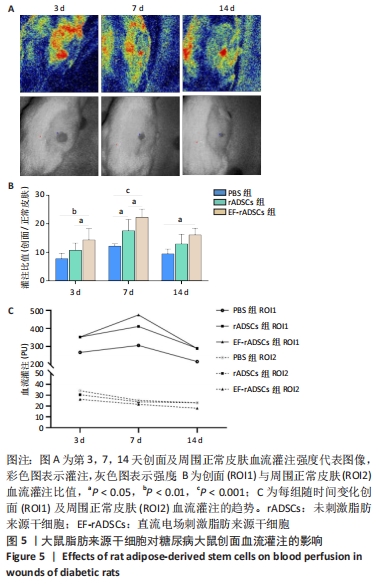
2.3.5 大鼠脂肪来源干细胞促进创面血流灌注 激光散斑对比成像仪测量不同时间点大鼠创面及周围正常皮肤血流灌注,结果显示,治疗后第3,7,14天,直流电场刺激脂肪来源干细胞组创面与周围正常皮肤血流灌注比值均高于PBS组(P < 0.05)。与未刺激脂肪来源干细胞组相比,第3,7天时直流电场刺激脂肪来源干细胞组创面血流灌注明显增高(P < 0.05),第14天时两组则无明显差异。未刺激脂肪来源干细胞组在第7天也表现出较PBS组更高的血流灌注(P < 0.05),而在第3,14天时则无统计学意义。此外,3组大鼠的血流灌注在第3天时稍低,第7天达到峰值,随后出现不同程度下降,表现出相同的趋势,见图5。"
| [1] OLSSON M, JÄRBRINK K, DIVAKAR U, et al. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019;27(1):114-125. [2] SUN X, NI P, WU M, et al. A Clinicoepidemiological Profile of Chronic Wounds in Wound Healing Department in Shanghai. Int J Low Extrem Wounds. 2017;16(1):36-44. [3] KERN S, EICHLER H, STOEVE J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294-1301. [4] ENGELA AU, BAAN CC, PEETERS AM, et al. Interaction between adipose tissue-derived mesenchymal stem cells and regulatory T-cells. Cell Transplant. 2013;22(1):41-54. [5] CAWTHORN WP, SCHELLER EL, MACDOUGALD OA. Adipose tissue stem cells: the great WAT hope. Trends Endocrinol Metab. 2012;23(6):270-277. [6] PUISSANT B, BARREAU C, BOURIN P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129(1):118-129. [7] LU C, KOLBENSCHLAG J, NÜSSLER AK, et al. Direct Current Electrical Fields Improve Experimental Wound Healing by Activation of Cytokine Secretion and Erk1/2 Pathway Stimulation. Life (Basel). 2021;11(11): 1195. [8] 刘兰,刘星可,刘梦嫦,等.直流电场对人脂肪间充质干细胞增殖和干细胞生物学特性的影响 [J]. 第三军医大学学报,2021,43(11): 1010-1017. [9] HOLM JS, TOYSERKANI NM, SORENSEN JA. Adipose-derived stem cells for treatment of chronic ulcers: current status. Stem Cell Res Ther. 2018;9(1):142. [10] SUN Y, SONG L, ZHANG Y, et al. Adipose stem cells from type 2 diabetic mice exhibit therapeutic potential in wound healing. Stem Cell Res Ther. 2020;11(1):298. [11] STOJANOVIĆ S, NAJMAN S. The Effect of Conditioned Media of Stem Cells Derived from Lipoma and Adipose Tissue on Macrophages’ Response and Wound Healing in Indirect Co-culture System In Vitro. Int J Mol Sci. 2019;20(7):1671. [12] KLAR AS, MICHALAK-MIĆKA K, BIEDERMANN T, et al. Characterization of M1 and M2 polarization of macrophages in vascularized human dermo-epidermal skin substitutes in vivo. Pediatr Surg Int. 2018;34(2): 129-135. [13] ZOMER HD, JEREMIAS TDS, RATNER B, et al. Mesenchymal stromal cells from dermal and adipose tissues induce macrophage polarization to a pro-repair phenotype and improve skin wound healing. Cytotherapy. 2020;22(5):247-260. [14] MAZINI L, ROCHETTE L, ADMOU B, et al. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int J Mol Sci. 2020;21(4):1306. [15] ZHOU X, NING K, LING B, et al. Multiple Injections of Autologous Adipose-Derived Stem Cells Accelerate the Burn Wound Healing Process and Promote Blood Vessel Regeneration in a Rat Model. Stem Cells Dev. 2019;28(21):1463-1472. [16] ALEXANDRUSHKINA N, NIMIRITSKY P, EREMICHEV R, et al. Cell Sheets from Adipose Tissue MSC Induce Healing of Pressure Ulcer and Prevent Fibrosis via Trigger Effects on Granulation Tissue Growth and Vascularization. Int J Mol Sci. 2020;21(15):5567. [17] HUANG SP, HUANG CH, SHYU JF, et al. Promotion of wound healing using adipose-derived stem cells in radiation ulcer of a rat model. J Biomed Sci. 2013;20(1):51. [18] GENG Y, YANG J, LI S, et al. Chyloid Fat Carried Adipose-Derived Mesenchymal Stem Cells Accelerate Wound Healing Via Promoting Angiogenesis. Ann Plast Surg. 2021;87(4):472-477. [19] NAMBU M, KISHIMOTO S, NAKAMURA S, et al. Accelerated wound healing in healing-impaired db/db mice by autologous adipose tissue-derived stromal cells combined with atelocollagen matrix. Ann Plast Surg. 2009;62(3):317-321. [20] AJIT A, SANTHOSH KUMAR TR, KRISHNAN LK. Engineered Human Adipose-Derived Stem Cells Inducing Endothelial Lineage and Angiogenic Response. Tissue Eng Part C Methods. 2019;25(3):148-159. [21] QIAN L, PI L, FANG BR, et al. Adipose mesenchymal stem cell-derived exosomes accelerate skin wound healing via the lncRNA H19/miR-19b/SOX9 axis. Lab Invest. 2021;101(9):1254-1266. [22] RODRIGUEZ J, BOUCHER F, LEQUEUX C, et al. Intradermal injection of human adipose-derived stem cells accelerates skin wound healing in nude mice. Stem Cell Res Ther. 2015;6:241. [23] LOVE MR, PALEE S, CHATTIPAKORN SC, et al. Effects of electrical stimulation on cell proliferation and apoptosis. J Cell Physiol. 2018; 233(3):1860-1876. [24] BROUGHTON G 2ND, JANIS JE, ATTINGER CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e-S-32e-S. [25] 颜洪.着眼瘢痕防治,促进创面愈合 [J].第三军医大学学报,2021, 43(11):997-1002. [26] WILKINSON HN, HARDMAN MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9):200223. [27] WU Y, CHEN L, SCOTT PG, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648-2659. [28] NIE C, YANG D, MORRIS SF. Local delivery of adipose-derived stem cells via acellular dermal matrix as a scaffold: a new promising strategy to accelerate wound healing. Med Hypotheses. 2009;72(6):679-682. [29] HUANG S, HU Z, WANG P, et al. Rat epidermal stem cells promote the angiogenesis of full-thickness wounds. Stem Cell Res Ther. 2020; 11(1):344. [30] DOMINICI M, LE BLANC K, MUELLER I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8(4):315-317. [31] BAO P, KODRA A, TOMIC-CANIC M, et al. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153(2):347-358. [32] BATES DO, JONES RO. The role of vascular endothelial growth factor in wound healing. Int J Low Extrem Wounds. 2003;2(2):107-120. [33] JOHNSON KE, WILGUS TA. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv Wound Care (New Rochelle). 2014;3(10):647-661. [34] CHUNG AS, FERRARA N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol. 2011;27:563-584. [35] ONG HT, DILLEY RJ. Novel non-angiogenic role for mesenchymal stem cell-derived vascular endothelial growth factor on keratinocytes during wound healing. Cytokine Growth Factor Rev. 2018;44:69-79. [36] ZHANG P, LU J, JING Y, et al. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. 2017; 49(2):106-116. [37] YANG M, SHENG L, ZHANG TR, et al. Stem cell therapy for lower extremity diabetic ulcers: where do we stand? Biomed Res Int. 2013; 2013:462179. [38] SORG H, TILKORN DJ, MIRASTSCHIJSKI U, et al. Panta Rhei: Neovascularization, Angiogenesis and Nutritive Perfusion in Wound Healing. Eur Surg Res. 2018;59(3-4):232-241. [39] REGE A, THAKOR NV, RHIE K, et al. In vivo laser speckle imaging reveals microvascular remodeling and hemodynamic changes during wound healing angiogenesis. Angiogenesis. 2012;15(1):87-98. |
| [1] | Zhong Yizheng, Huang Peizhen, Cai Qunbin, Zheng Liqin, He Xingpeng, Dong Hang. Microstructural indexes that determine the trabecular bone maximum stress of micro-finite element models [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1313-1318. |
| [2] | Cao Sheng, Kong Lingwei, Xu Kun, Sun Zhijie. Correlation of cervical sagittal force line parameters with degenerative segment and Pfirrmann classification in patients with cervical intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1319-1324. |
| [3] | Ke Yuqi, Chen Changjian, Wu Hao, Zheng Lianjie. Comparison of 12-month follow-up results of primary total hip arthroplasty between modified direct anterior approach and direct anterior approach [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1377-1382. |
| [4] | Zhang Lichuang, Gao Huali, Wang Jingchao, Lin Huijun, Wu Chonggui, Ma Yinghui, Huang Yunfei, Fang Xue, Zhai Weitao. Effect of tendon manipulation with equal emphasis on muscles and bones on accelerating the functional rehabilitation of quadriceps femoris after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1383-1389. |
| [5] | Du Xueting, Zhang Xiaodong, Chen Yanjun, Wang Mei, Chen Wubiao, Huang Wenhua. Application of compressed sensing technology in two-dimensional magnetic resonance imaging of the ankle joint [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1396-1402. |
| [6] | You Zhengqiu, Zhang Zhongzu, Wang Qunbo. Early symptomatic intervertebral disc pseudocysts after discectomy detected on MRI [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1403-1409. |
| [7] | Li Chao, Zhang Peipei, Xu Mengting, Li Linlin, Ding Jiangtao, Liu Xihua, Bi Hongyan. Respiratory training improves morphological changes of the multifidus muscle in patients with chronic nonspecific lower back pain assessed by musculoskeletal ultrasound [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1417-1421. |
| [8] | He Yinhao, Li Xiaosheng, Chen Hongwen, Chen Tiezhu. 3D printed porous tantalum metal in the treatment of developmental dysplasia of the hip: current status and application prospect [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1455-1461. |
| [9] | Jiang Xiaocheng, Shi Lu, Wang Yinbin, Li Qiujiang, Xi Chuangzhen, Ma Zefeng, Cai Lijun. Systematical evaluation of bone fusion rate after interbody fusion in patients with osteoporosis and lumbar degenerative disease treated with teriparatide [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1427-1433. |
| [10] | Sun Jiajia, Zhu Haidi, Lu Yun, Zhang Kai. Comparison of bone metabolism markers between type 2 diabetes mellitus and non-type 2 diabetes mellitus patients with hip fracture [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1156-1160. |
| [11] | Li Mengfei, Zhang Hong, Zhao Shaojian, Yin Guanghao, Wang Qibao. Expression of forkhead box protein 3 in refractory periapical periodontitis in rats with Enterococcus faecalis infection [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1187-1192. |
| [12] | Lian Shilin, Zhang Yan, Jiang Qiang, Zhang Hanshuo, Li Tusheng, Ding Yu. Interventional effects of whole blood and platelet-rich plasma with different preparation methods on nucleus pulposus cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1199-1204. |
| [13] | Wu Dongzhe, Gao Xiaolin, Li Chuangtao, Wang Hao. Constructing the prediction model of maximal oxygen uptake by back-propagation neural network based on the cardiorespiratory optimal point [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1224-1231. |
| [14] | Yang Jiujie, Li Zhi, Wang Shujie, Tian Ye, Zhao Wei. Intraoperative neurophysiological monitoring of functional changes following durotomy with decompression for acute spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1232-1236. |
| [15] | Wang Ji, Zhang Min, Yang Zhongya, Zhang Long. A review of physical activity intervention in type 2 diabetes mellitus with sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1272-1277. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||