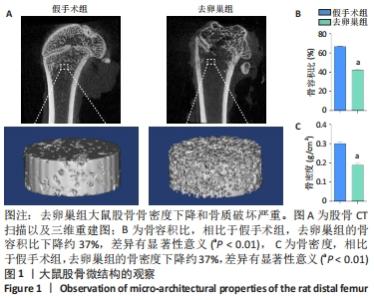
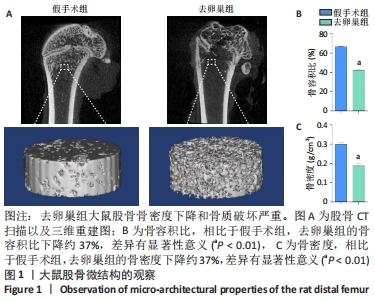
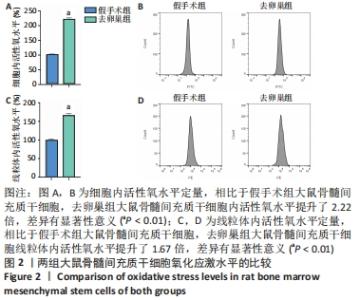
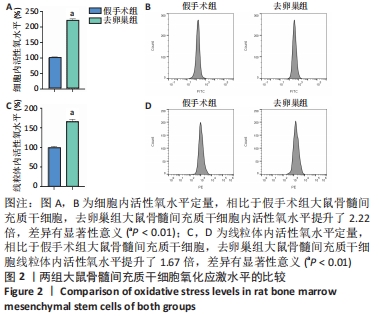
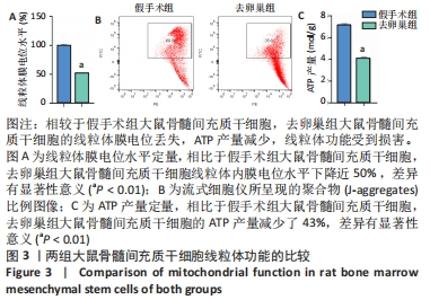
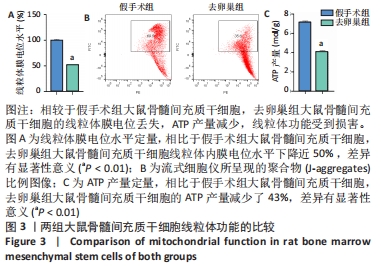
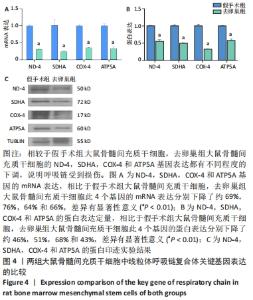
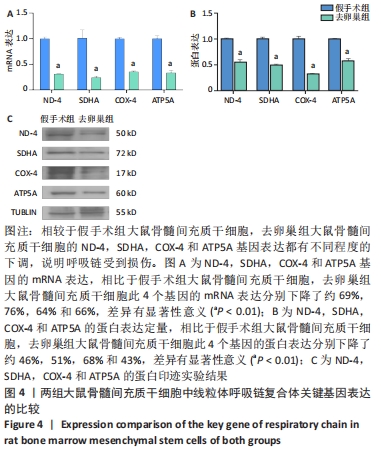
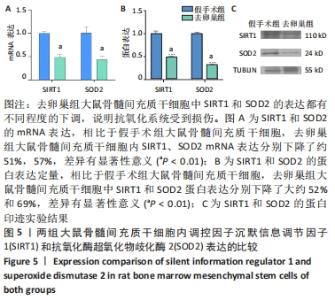
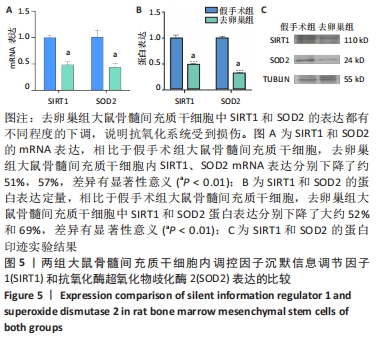
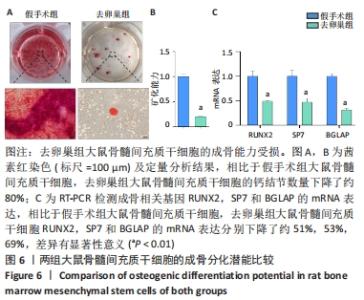
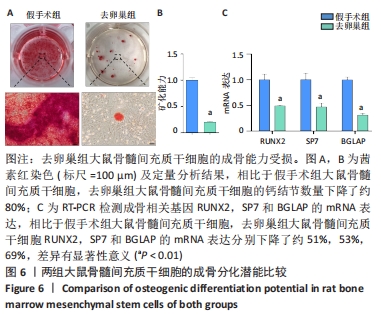
Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (31): 4921-4927.doi: 10.12307/2022.727
Mitochondrial dysfunction affects osteogenic differentiation potential of bone marrow mesenchymal stem cells
Gu Chao1, 2, Chen Weikai1, 2, Liu Tao1, Yang Huilin1, 2, He Fan1, 2
- 1Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China; 2Institute of Orthopedics, Soochow University, Suzhou 215007, Jiangsu Province, China
-
Received:2021-11-04Accepted:2021-12-15Online:2022-11-08Published:2022-04-24 -
Contact:He Fan, PhD, Researcher, Doctoral supervisor, Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China; Institute of Orthopedics, Soochow University, Suzhou 215007, Jiangsu Province, China -
About author:Gu Chao, Master candidate, Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China; Institute of Orthopedics, Soochow University, Suzhou 215007, Jiangsu Province, China -
Supported by:National Natural Science Foundation of China, No. 31771063 (to HF); National Natural Science Foundation of China, No. 82072476 (to LT); Natural Science Foundation of Jiangsu Province, No. BK20191173 (to LT)
CLC Number:
Cite this article
Gu Chao, Chen Weikai, Liu Tao, Yang Huilin, He Fan. Mitochondrial dysfunction affects osteogenic differentiation potential of bone marrow mesenchymal stem cells[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(31): 4921-4927.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] LANGDAHL BL. Overview of treatment approaches to osteoporosis. Br J Pharmacol. 2021;178(9):1891-1906. [2] ANAM AK, INSOGNA K. Update on Osteoporosis Screening and Management. Med Clin North Am. 2021;105(6):1117-1134. [3] RACHNER TD, KHOSLA S, HOFBAUER LC. Osteoporosis: now and the future. Lancet. 2011;377(9773):1276-1287. [4] SALARI N, GHASEMI H, MOHAMMADI L, et al. The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J Orthop Surg Res. 2021;16(1):609. [5] CHENG X, ZHAO K, ZHA X, et al. Opportunistic Screening Using Low-Dose CT and the Prevalence of Osteoporosis in China: A Nationwide, Multicenter Study. J Bone Miner Res. 2021;36(3):427-435. [6] LEE NK, CHOI YG, BAIK JY, et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005; 106(3):852-859. [7] HO PJ, YEN ML, TANG BC, et al. H2O2 accumulation mediates differentiation capacity alteration, but not proliferative decline, in senescent human fetal mesenchymal stem cells. Antioxid Redox Signal. 2013;18(15):1895-1905. [8] ZHANG YB, ZHONG ZM, HOU G, et al. Involvement of oxidative stress in age-related bone loss. J Surg Res. 2011;169(1):e37-42. [9] KALYANARAMAN B. Teaching the basics of repurposing mitochondria-targeted drugs: From Parkinson’s disease to cancer and back to Parkinson’s disease. Redox Biol. 2020;36:101665. [10] MENDIVIL-PEREZ M, SOTO-MERCADO V, GUERRA-LIBRERO A, et al. Melatonin enhances neural stem cell differentiation and engraftment by increasing mitochondrial function. J Pineal Res. 2017;63(2). doi: 10.1111/jpi.12415. [11] KUNG HC, LIN KJ, KUNG CT, et al. Oxidative Stress, Mitochondrial Dysfunction, and Neuroprotection of Polyphenols with Respect to Resveratrol in Parkinson’s Disease. Biomedicines. 2021;9(8):918. [12] SHARES BH, BUSCH M, WHITE N, et al. Active mitochondria support osteogenic differentiation by stimulating β-catenin acetylation. J Biol Chem. 2018;293(41):16019-16027. [13] GRAYSON WL, ZHAO F, BUNNELL B, et al. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358(3):948-953. [14] WANG C, MENG H, WANG X, et al. Differentiation of Bone Marrow Mesenchymal Stem Cells in Osteoblasts and Adipocytes and its Role in Treatment of Osteoporosis. Med Sci Monit. 2016;22:226-233. [15] KOMAROVA SV, ATAULLAKHANOV FI, GLOBUS RK. Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am J Physiol Cell Physiol. 2000;279(4):C1220-1229. [16] CHA Y, HAN MJ, CHA HJ, et al. Metabolic control of primed human pluripotent stem cell fate and function by the miR-200c-SIRT2 axis. Nat Cell Biol. 2017;19(5):445-456. [17] DI MEO S, REED TT, VENDITTI P, et al. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid Med Cell Longev. 2016; 2016:1245049. [18] WANG FS, WU RW, CHEN YS, et al. Biophysical Modulation of the Mitochondrial Metabolism and Redox in Bone Homeostasis and Osteoporosis: How Biophysics Converts into Bioenergetics. Antioxidants (Basel). 2021;10(9):1394. [19] YANG S, FESKANICH D, WILLETT WC, et al. Association between global biomarkers of oxidative stress and hip fracture in postmenopausal women: a prospective study. J Bone Miner Res. 2014;29(12):2577-2583. [20] LANGDAHL JH, FREDERIKSEN AL, HANSEN SJ, et al. Mitochondrial Point Mutation m.3243A>G Associates With Lower Bone Mineral Density, Thinner Cortices, and Reduced Bone Strength: A Case-Control Study. J Bone Miner Res. 2017;32(10):2041-2048. [21] OZGOCMEN S, KAYA H, FADILLIOGLU E, et al. Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol Cell Biochem. 2007;295(1-2):45-52. [22] ZINNUROGLU M, DINCEL AS, KOSOVA F, et al. Prospective evaluation of free radicals and antioxidant activity following 6-month risedronate treatment in patients with postmenopausal osteoporosis. Rheumatol Int. 2012;32(4):875-880. [23] TANG X, MA S, LI Y, et al. Evaluating the Activity of Sodium Butyrate to Prevent Osteoporosis in Rats by Promoting Osteal GSK-3β/Nrf2 Signaling and Mitochondrial Function. J Agric Food Chem. 2020;68(24): 6588-6603. [24] KOBAYASHI K, NOJIRI H, SAITA Y, et al. Mitochondrial superoxide in osteocytes perturbs canalicular networks in the setting of age-related osteoporosis. Sci Rep. 2015;5:9148. [25] CHEN CT, SHIH YR, KUO TK, et al. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26(4):960-968. [26] DICKIE LJ, AZIZ AM, SAVIC S, et al. Involvement of X-box binding protein 1 and reactive oxygen species pathways in the pathogenesis of tumour necrosis factor receptor-associated periodic syndrome. Ann Rheum Dis. 2012;71(12):2035-2043. [27] ANDRÉ L, GOUZI F, THIREAU J, et al. Carbon monoxide exposure enhances arrhythmia after cardiac stress: involvement of oxidative stress. Basic Res Cardiol. 2011;106(6):1235-1246. [28] SATO T, SAITO T, SAEGUSA N, et al. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: a mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation. 2005;111(2): 198-203. [29] ZHANG F, CUI J, LIU X, et al. Correction to: Roles of microRNA-34a targeting SIRT1 in mesenchymal stem cells. Stem Cell Res Ther. 2020; 11(1):331. [30] LI HJ, SUN XM, LI ZK, et al. LncRNA UCA1 Promotes Mitochondrial Function of Bladder Cancer via the MiR-195/ARL2 Signaling Pathway. Cell Physiol Biochem. 2017;43(6):2548-2561. [31] ZHOU J, ZHANG F, MENG H, et al. Introducing extra NADPH consumption ability significantly increases the photosynthetic efficiency and biomass production of cyanobacteria. Metab Eng. 2016;38:217-227. [32] LV YJ, YANG Y, SUI BD, et al. Resveratrol counteracts bone loss via mitofilin-mediated osteogenic improvement of mesenchymal stem cells in senescence-accelerated mice. Theranostics. 2018;8(9):2387-2406. [33] EBERT R, ULMER M, ZECK S, et al. Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro. Stem Cells. 2006;24(5):1226-1235. [34] NDI M, MARIN-BUERA L, SALVATORI R, et al. Biogenesis of the bc(1) Complex of the Mitochondrial Respiratory Chain. J Mol Biol. 2018; 430(21):3892-3905. [35] GELDON S, FERNÁNDEZ-VIZARRA E, TOKATLIDIS K. Redox-Mediated Regulation of Mitochondrial Biogenesis, Dynamics, and Respiratory Chain Assembly in Yeast and Human Cells. Front Cell Dev Biol. 2021;9: 720656. [36] HE J, LIU X, SU C, et al. Inhibition of Mitochondrial Oxidative Damage Improves Reendothelialization Capacity of Endothelial Progenitor Cells via SIRT3 (Sirtuin 3)-Enhanced SOD2 (Superoxide Dismutase 2) Deacetylation in Hypertension. Arterioscler Thromb Vasc Biol. 2019; 39(8):1682-1698. [37] TSAI YT, YEH HY, CHAO CT, et al. Superoxide Dismutase 2 (SOD2) in Vascular Calcification: A Focus on Vascular Smooth Muscle Cells, Calcification Pathogenesis, and Therapeutic Strategies. Oxid Med Cell Longev. 2021;2021:6675548. [38] CHEN W, CHEN X, CHEN AC, et al. Melatonin restores the osteoporosis-impaired osteogenic potential of bone marrow mesenchymal stem cells by preserving SIRT1-mediated intracellular antioxidant properties. Free Radic Biol Med. 2020;146:92-106. [39] GAO J, FENG Z, WANG X, et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ. 2018;25(2):229-240. |
| [1] | Cui Wei, Cui Di, Ouyang Ting, Li Xiang, Wei Huiting, Xue Weiyue, Zhou Gang, Qiu Ye. Inhibiting NOX alleviates alcoholic liver damage and lipid metabolism disorder [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(在线): 1-8. |
| [2] | Jiang Huanchang, Zhang Zhaofei, Liang De, Jiang Xiaobing, Yang Xiaodong, Liu Zhixiang. Comparison of advantages between unilateral multidirectional curved and straight vertebroplasty in the treatment of thoracolumbar osteoporotic vertebral compression fracture [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1407-1411. |
| [3] | Zhu Chan, Han Xuke, Yao Chengjiao, Zhou Qian, Zhang Qiang, Chen Qiu. Human salivary components and osteoporosis/osteopenia [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1439-1444. |
| [4] | Li Wei, Zhu Hanmin, Wang Xin, Gao Xue, Cui Jing, Liu Yuxin, Huang Shuming. Effect of Zuogui Wan on bone morphogenetic protein 2 signaling pathway in ovariectomized osteoporosis mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1173-1179. |
| [5] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [6] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [7] | Gao Yujin, Peng Shuanglin, Ma Zhichao, Lu Shi, Cao Huayue, Wang Lang, Xiao Jingang. Osteogenic ability of adipose stem cells in diabetic osteoporosis mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 999-1004. |
| [8] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [9] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [10] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [11] | Peng Kun. Improvement of the treatment effect of osteoporotic fractures: research status and strategy analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 980-984. |
| [12] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [13] | Ou Liang, Kong Dezhong, Xu Daoqing, Ni Jing, Fu Xingqian, Huang Weichen. Comparative clinical efficacy of polymethyl methacrylate and self-solidifying calcium phosphate cement in vertebroplasty: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 649-656. |
| [14] | Shen Song, Xu Bin. Diffuse distribution of bone cement in percutaneous vertebroplasty reduces the incidence of refracture of adjacent vertebral bodies [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 499-503. |
| [15] | Sun Xirao, Bao Jiaxin, Wang Chengyue. Construction of chitosan/mineralized collagen porous scaffold, osteogenic differentiation in vitro and biocompatibility [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(34): 5498-5503. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||