Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (20): 3133-3140.doi: 10.12307/2022.610
Previous Articles Next Articles
Mechanism of osteoarthritis induced chondrocyte apoptosis and extracellular matrix degradation
Wang Wei1, Tang Xiangyu2, Yi Zhiqian1, Liu Zhaoxu1
- 1Department of Orthopedics, 2Department of Radiology, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China
-
Received:2021-04-22Accepted:2021-06-01Online:2022-07-18Published:2022-01-18 -
Contact:Liu Zhaoxu, MD, Associate chief physician, Department of Orthopedics, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China -
About author:Wang Wei, MD, Attending physician, Department of Orthopedics, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China -
Supported by:the National Natural Science Foundation of China, No. 51877097 (to LZX)
CLC Number:
Cite this article
Wang Wei, Tang Xiangyu, Yi Zhiqian, Liu Zhaoxu. Mechanism of osteoarthritis induced chondrocyte apoptosis and extracellular matrix degradation[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(20): 3133-3140.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
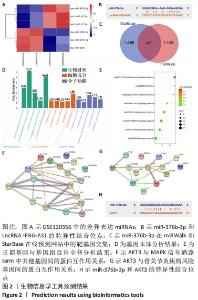
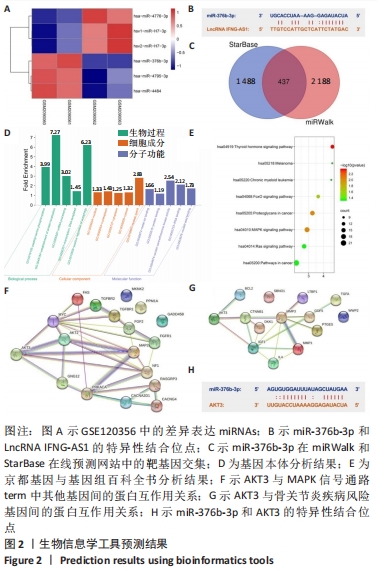
2.1 生物信息学工具预测结果 结合GSE120356芯片分析结果,取表达差异前6位的miRNA绘制热图。结果发现miR-376b-3p在骨关节炎患者关节软骨组织中的表达水平显著低于腰间椎盘突出患者,见图2A。通过LncRNASNP2数据库发现miR-376b-3p和LncRNA IFNG-AS1具有特异性结合位点,见图2B,IFNG-AS1在骨关节炎中的作用尚不清楚,有待进一步探究。在miRWalk和StarBase靶向关系预测网站找到miR-376b-3p的下游靶基因并绘制Venn图,见图2C,共有437个交集靶基因,将这些靶基因经DAVID网站进行基因本体和京都基因与基因组百科全书富集分析,根据显著富集程度分别从Biological process、Cellular component和Molecular function按照显著性选取前5个term绘制富集柱状图,见图2D,结果显示上述基因在基因本体分析中没有找到和此次研究相关的通路。 随后观察京都基因与基因组百科全书分析结果,发现上述基因在癌症相关通路(Pathways in cancer)、Ras信号通路和MAPK信号通路显著富集,见图2E,MAPK信号通路被证实是骨关节炎发病过程中的重要通路,因此将MAPK信号通路的term作为目标基因选取范围。借助STRING和DisGeNET在线工具发现AKT3在MAPK信号通路中与其他基因的关联性较强,见图2F,且和骨关节炎疾病风险基因连接紧密,见图2G。此外,鉴于AKT3在体外的骨关节炎中发挥恶性作用,将AKT3纳入此次研究。"
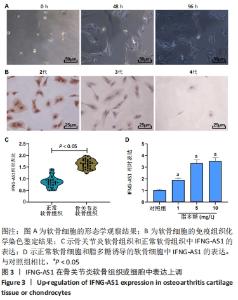
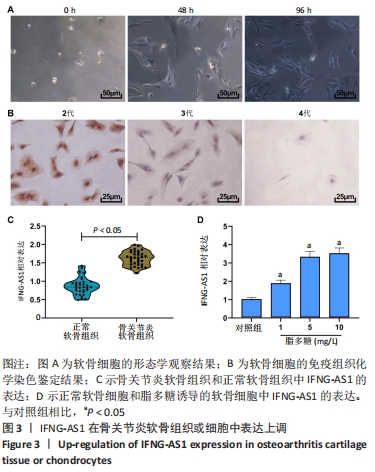
2.2 IFNG-AS1在骨关节炎软骨组织和脂多糖诱导的软骨细胞中表达升高 形态学观察显示,分离的软骨细胞呈球形,大小均匀,易于悬浮,几乎不贴壁;培养48 h后,软骨细胞分化为椭圆形和梭形,细胞核细长,胞浆丰富;培养96 h后,软骨细胞生长迅速,分裂成更多的梭形细胞和多边形细胞,相互连接形成更多的细胞集落,见图3A。 免疫组化染色显示,在软骨细胞传代培养过程中,第2代软骨细胞色素沉着良好,第3代软骨细胞色素沉着较少,Ⅱ型胶原阳性软骨细胞和第4代软骨细胞色素沉着最少,见图3B。 用实时荧光定量PCR方法分析了33例骨关节炎软骨组织和正常软骨组织中IFNG-AS1的表达水平,发现IFNG-AS1在骨关节炎软骨组织中表达增加(均P < 0.05),见图3C。随后,在脂多糖诱导的软骨细胞中检测IFNG-AS1水平,结果显示,经脂多糖处理后,IFNG-AS1水平以剂量依赖性方式显著上调(均P < 0.05),见图3D。提示IFNG-AS1可能参与了骨关节炎的进展。"
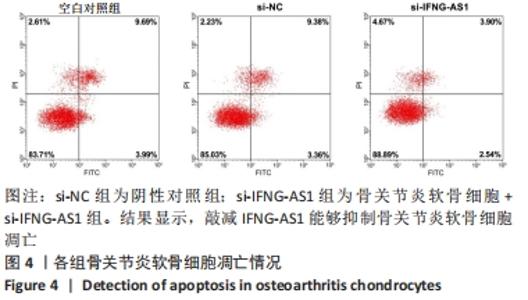
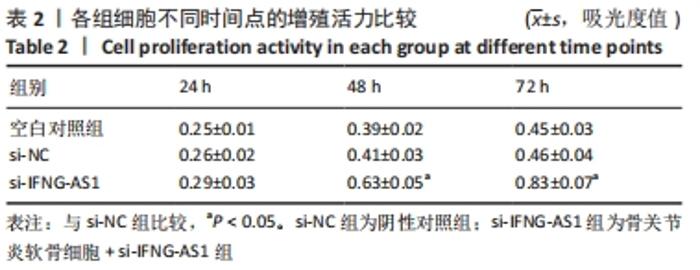
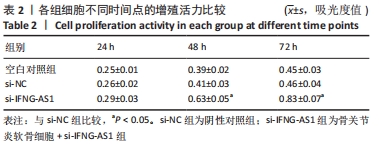
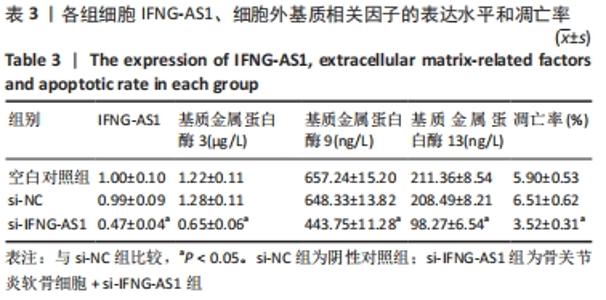
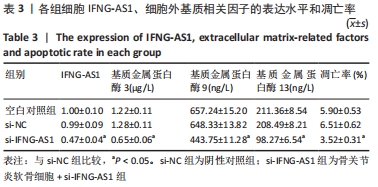
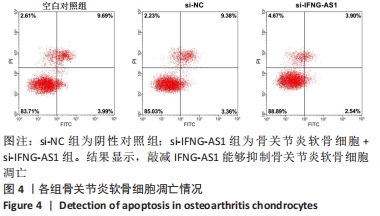
2.3 IFNG-AS1基因敲减抑制脂多糖诱导的软骨细胞损伤 为了进一步探讨IFNG-AS1在脂多糖诱导的软骨细胞生长中的作用,将si-IFNG-AS1转染到脂多糖(5 mg/L)刺激的软骨细胞中,进行IFNG-AS1敲减。实时荧光定量PCR检测表明,脂多糖处理(空白对照组)上调了IFNG-AS1的表达,通过转染si-IFNG-AS1显著下调了IFNG-AS1的表达(均P < 0.05)。之后进行CCK-8检测以评估细胞活性,结果显示,脂多糖处理显著抑制细胞活力,si-IFNG-AS1组在48 h(0.63±0.05)和72 h(0.83±0.07)时的细胞增殖活力均显著高于同时间点的si-NC组(0.41±0.03)和(0.46±0.04)(均P < 0.05)。通过流式细胞仪分析,发现IFNG-AS1基因敲减后的细胞凋亡率(3.52±0.31)%明显低于si-NC组(6.51±0.62)%(P < 0.05)。检测细胞外基质相关因子基质金属蛋白酶3,9,13的水平,结果表明,IFNG-AS1下调逆转了脂多糖处理对软骨细胞中基质金属蛋白酶水平的影响,基质金属蛋白酶3,9,13的表达水平均较si-NC组明显降低(均P < 0.05)。这些数据表明IFNG-AS1的缺失抑制了脂多糖诱导的骨关节炎软骨细胞凋亡和细胞外基质降解,见图4及表2,3。"
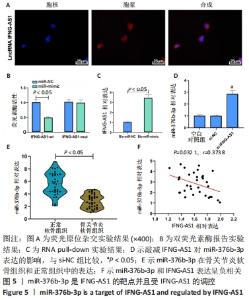
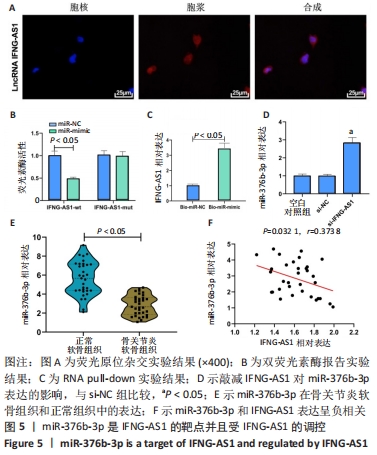
2.4 IFNG-AS1直接下调miR-376b-3p的表达 首先用荧光原位杂交法检测了IFNG-AS1的亚细胞定位,发现其主要定位于细胞质,见图5A。之后采用双荧光素酶报告实验验证了IFNG-AS1和miR-376b-3p之间的互作用关系,见图5B,与miR-NC和IFNG-AS1-wt共转染组相比,共转染miR-mimic和IFNG-AS1-wt组的荧光素酶活性被抑制(P < 0.05),提示miR-376b-3p与IFNG-AS1存在靶向关系。同时,这种相互作用通过RNA pull-down实验得到证实,见图5C。然后,将IFNG-AS1或si-IFNG-AS1转染到软骨细胞,探讨IFNG-AS1对miR-376b-3p表达的影响,证实IFNG-AS1敲减显著上调miR-376b-3p水平,见图5D。此外,发现相对于正常软骨组织,骨关节炎软骨组织中miR-376b-3p的表达下调,见图5E,与IFNG-AS1的相应表达呈负相关性,见图5F。总之,IFNG-AS1作为miR-376b-3p海绵,对miR-376b-3p的表达有负性调节作用。"
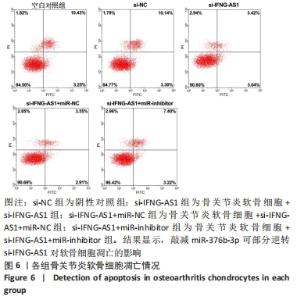
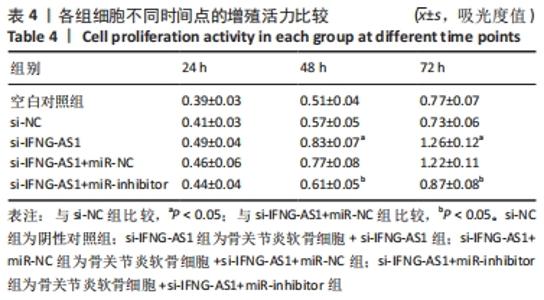
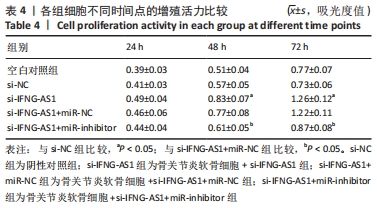
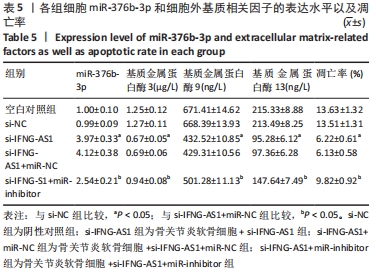
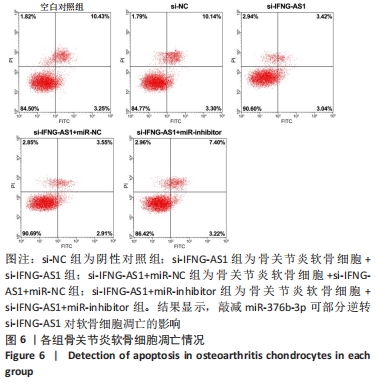
2.5 IFNG-AS1调节miR-376b-3p的表达促进脂多糖诱导的骨关节炎软骨细胞损伤 为探讨miR-376b-3p和IFNG-AS1对脂多糖作用后软骨细胞生长的作用。用脂多糖处理软骨细胞后分别转染si-NC、si-IFNG-AS1、si-IFNG-AS1+miR-NC或si-IFNG-AS1+miR-inhibitor。实时荧光定量PCR分析证实,较si-NC组(0.99±0.09)相比,si-IFNG-AS1组(3.97±0.33)的miR-376b-3p表达水平显著上调,在加入miR-inhibitor(2.54±0.21)后下调(均P < 0.05)。之后评估细胞活力,si-IFNG-AS1+miR-inhibitor的转染使细胞在48 h和72 h时的细胞增殖活力显著低于同时间点si-IFNG-AS1+miR-NC组(均P < 0.05)。此外,相对于si-IFNG-AS1+miR-NC组的细胞凋亡率(6.13±0.58)%,si-IFNG-AS1+miR-inhibitor细胞凋亡率(9.82±0.92)%上调(P < 0.05)。此外,此次研究发现miR-376b-3p的下调减弱了IFNG-AS1基因敲减对细胞外基质相关因子水平的影响,IFNG-AS1基因敲减降低了基质金属蛋白酶3,9,13的表达水平,然后这种作用被miR-376b-3p inhibitor部分逆转(均P < 0.05)。以上结果表明,IFNG-AS1通过调节miR-376b-3p的表达来介导脂多糖诱导的软骨细胞凋亡和细胞外基质降解,见图6及表4,5。"
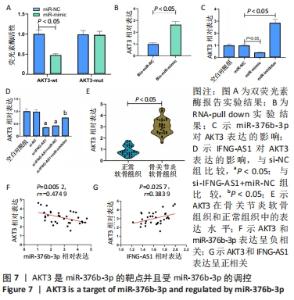
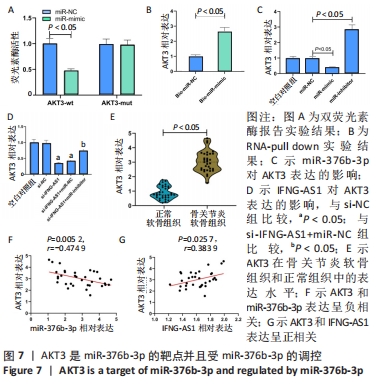
2.6 miR-376b-3p靶向结合并下调AKT3的水平 双荧光素酶报告分析被用于证实miR-376b-3p和AKT3的相互作用关系,结果表明,miR-376b-3p能显著降低AKT3-wt的荧光素酶活性,但对AKT3-mut无明显影响,见图7A。RNA pull-down实验进一步验证了二者间的靶向结合关系,见图7B。之后研究miR-376b-3p对软骨细胞AKT3表达的影响,见图7C,AKT3的mRNA水平被miR-376b-3p过度表达下调,被miR-376b-3p敲减上调(均P < 0.05),提示miR-376b-3p能够靶向抑制AKT3的表达。此外,此次研究证实了IFNG-AS1敲减能够下调AKT3水平,而miR-inhibitor的同步上调削弱了这种变化,见图7D,提示IFNG-AS1能够通过调控miR-376b-3p的表达进而影响软骨细胞中AKT3的水平。此外,在骨关节炎软骨组织中观察到AKT3表达增加,见图7E。如预期,AKT3水平与miR-376b-3p水平呈负相关(P=0.005 2,r=-0.474 9),与IFNG-AS1水平呈正相关(P=0.025 7,r=0.383 9),见图7F,G。因此,IFNG-AS1通过调节miR-376b-3p的表达来调节AKT3的表达水平。"
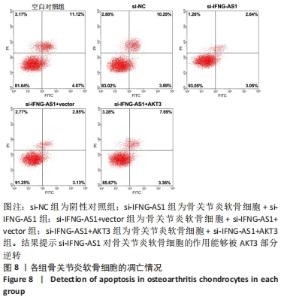
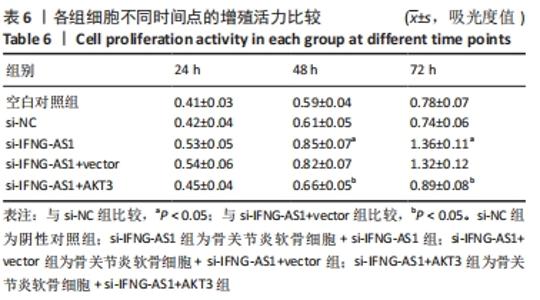
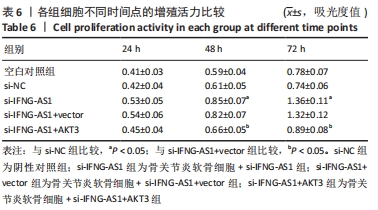
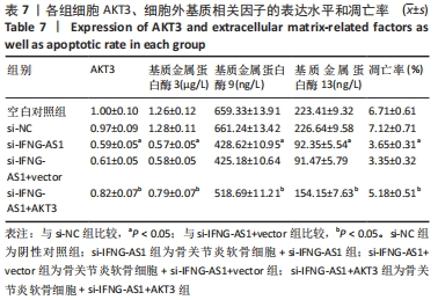
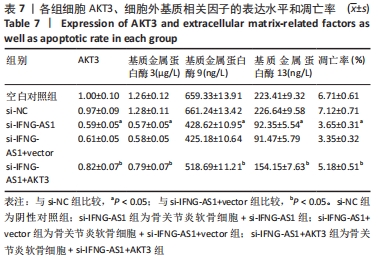
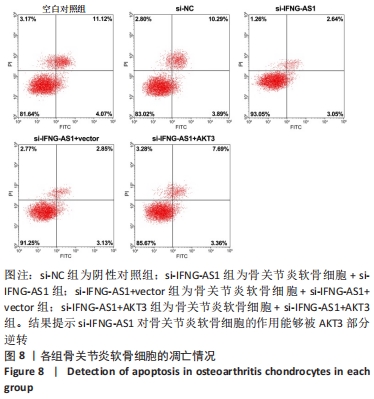
2.7 IFNG-AS1调节AKT3的水平促进脂多糖诱导的软骨细胞损伤 首先观察干预IFNG-AS1对AKT3表达的影响,结果显示,敲减IFNG-AS1能够显著抑制AKT3的表达。此外,相对于si-IFNG-AS1+vector组在48 h和72 h时的细胞活力, si-IFNG-AS1+AKT3组细胞活力显著降低(均P < 0.05)。流式细胞术检测结果显示,si-IFNG-AS1+AKT3组的细胞凋亡(5.18±0.51)%较si-IFNG-AS1+vector组(3.35±0.32)%显著增加(均P < 0.05)。基质金属蛋白酶的检测数据显示,si-IFNG-AS1+AKT3组的基质金属蛋白酶3,9,13表达水平较si-IFNG-AS1+vector组显著升高(均P < 0.05)。以上结果表明,IFNG-AS1通过调节AKT3的表达来介导脂多糖诱导的软骨细胞损伤,见图8及表6,7。"
| [1] Najar M,Martel-Pelletier J, Pelletier JP, et al. Mesenchymal Stromal Cell Immunology for Efficient and Safe Treatment of Osteoarthritis. Front Cell Dev Biol. 2020;8:567813. [2] Villas-Boas IM, Pidde G, Lichtenstein F, et al. PremolissemirufaHuman Chondrocyte Activation by Toxins From , an Amazon Rainforest Moth Caterpillar: Identifying an Osteoarthritis Signature.Front Immunol. 2020;11:2191. [3] Xin F, Wang H, Yuan F, et al. Platelet-Rich Plasma Combined with Alendronate Reduces Pain and Inflammation in Induced Osteoarthritis in Rats by Inhibiting the Nuclear Factor-Kappa B Signaling Pathway.Biomed Res Int. 2020;2020:8070295. [4] Shi L, Wang K, Yu J, et al. Relationship Between Magnetic Resonance T2-Mapping and Matrix Metalloproteinase 1,3 in Knee Osteoarthritis.Indian J Orthop. 2021;55(4):974-982. [5] Zhang G, Zhang Q, Zhu J, et al. LncRNA ARFRP1 knockdown inhibits LPS-induced the injury of chondrocytes by regulation of NF-κB pathway through modulating miR-15a-5p/TLR4 axis.Life Sci. 2020;261:118429. [6] Chen Y, Guo H, Li L, et al. Long Non-Coding RNA (lncRNA) Small Nucleolar RNA Host Gene 15 (SNHG15) Alleviates Osteoarthritis Progression by Regulation of Extracellular Matrix Homeostasis. Med Sci Monit. 2020;26:e923868. [7] Wang L,Yang M, Zhang C, et al. The protective effects of dehydrocostus lactone against TNF-α-induced degeneration of extracellular matrix (ECM) in SW1353 cells.Aging (Albany NY). 2020;12(17):17137-17149. [8] Song Y, Hao D, Jiang H, et al. Nrf2 Regulates CHI3L1 to Suppress Inflammation and Improve Post-Traumatic Osteoarthritis.J Inflamm Res. 2021;14:4079-4088. [9] Li L, Pang Y, Zhang L, et al. Triiodothyronine potentiates angiogenesis-related factor expression through PI3K/AKT signaling pathway in human osteoarthritic osteoblasts.Iran J Basic Med Sci. 2020;23(6):819-825. [10] Peng H, Ren S, Liu Y, et al. Elevated Expression of the Long Noncoding RNA IFNG-AS1 in the Peripheral Blood from Patients with Rheumatoid Arthritis.J Immunol Res. 2020;2020:6401978. [11] Huang B, Yu H, Li Y, et al. Upregulation of long noncoding TNFSF10 contributes to osteoarthritis progression through the miR-376-3p/FGFR1 axis.J Cell Biochem. 2019;120(12):19610-19620. [12] Zhang Y, Wang F, Chen G, et al. LncRNA MALAT1 promotes osteoarthritis by modulating miR-150-5p/AKT3 axis.Cell Biosci. 2019; 9: 54. [13] Houben CHM, Spruit MA, Luyten H, et al. Cluster-randomised trial of a nurse-led advance care planning session in patients with COPD and their loved ones. Thorax. 2019;74(4):328-336. [14] Li C, Cui L, Li S, et al. Long non-coding RNA Mirt2 interacts with long non-coding RNA IFNG-AS1 to regulate ulcerative colitis. Exp Ther Med. 2020;20(5):32. [15] Rankin CR, Shao L, Elliott J, et al. IFNG-AS1The IBD-associated long noncoding RNA regulates the balance between inflammatory and anti-inflammatory cytokine production after T-cell stimulation.Am J PhysiolGastrointest Liver Physiol. 2020;318(1):G34-G40. [16] Xu Y, Shao B. Circulating lncRNA IFNG-AS1 expression correlates with increased disease risk, higher disease severity and elevated inflammation in patients with coronary artery disease. J Clin Lab Anal. 2018;32(7):e22452. [17] Chen K, Fang H, Xu N. LncRNA LOXL1-AS1 is transcriptionally activated by JUND and contributes to osteoarthritis progression via targeting the miR-423-5p/KDM5C axis. Life Sci. 2020;258:118095. [18] Huang PY, Wu JG, Gu J, et al. Bioinformatics analysis of miRNA and mRNA expression profiles to reveal the key miRNAs and genes in osteoarthritis.J Orthop Surg Res. 2021;16(1):63. [19] Zhang C, Wang L, Yang J, et al. MicroRNA-33a-5p suppresses esophageal squamous cell carcinoma progression via regulation of lncRNA DANCR and ZEB1.Eur J Pharmacol. 2019;861:172590. [20] Litherland GJ, Dixon C, Lakey RL, et al. Synergistic collagenase expression and cartilage collagenolysis are phosphatidylinositol 3-kinase/Akt signaling-dependent.J Biol Chem. 2008;283(21):14221-14229. [21] Greene MA, Loeser RF. Function of the chondrocyte PI-3 kinase-Akt signaling pathway is stimulus dependent.Osteoarthritis Cartilage. 2015; 23(6):949-956. |
| [1] | Jin Tao, Liu Lin, Zhu Xiaoyan, Shi Yucong, Niu Jianxiong, Zhang Tongtong, Wu Shujin, Yang Qingshan. Osteoarthritis and mitochondrial abnormalities [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1452-1458. |
| [2] | Zhang Jichao, Dong Yuefu, Mou Zhifang, Zhang Zhen, Li Bingyan, Xu Xiangjun, Li Jiayi, Ren Meng, Dong Wanpeng. Finite element analysis of biomechanical changes in the osteoarthritis knee joint in different gait flexion angles [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1357-1361. |
| [3] | Wu Cong, Jia Quanzhong, Liu Lun. Relationship between transforming growth factor beta1 expression and chondrocyte migration in adult articular cartilage after fragmentation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1167-1172. |
| [4] | Wang Baojuan, Zheng Shuguang, Zhang Qi, Li Tianyang. Miao medicine fumigation can delay extracellular matrix destruction in a rabbit model of knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1180-1186. |
| [5] | Liu Dongcheng, Zhao Jijun, Zhou Zihong, Wu Zhaofeng, Yu Yinghao, Chen Yuhao, Feng Dehong. Comparison of different reference methods for force line correction in open wedge high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 827-831. |
| [6] | Zhou Jianguo, Liu Shiwei, Yuan Changhong, Bi Shengrong, Yang Guoping, Hu Weiquan, Liu Hui, Qian Rui. Total knee arthroplasty with posterior cruciate ligament retaining prosthesis in the treatment of knee osteoarthritis with knee valgus deformity [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 892-897. |
| [7] | He Junjun, Huang Zeling, Hong Zhenqiang. Interventional effect of Yanghe Decoction on synovial inflammation in a rabbit model of early knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 694-699. |
| [8] | Lin Xuchen, Zhu Hainian, Wang Zengshun, Qi Tengmin, Liu Limin, Suonan Angxiu. Effect of xanthohumol on inflammatory factors and articular cartilage in a mouse mode of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 676-681. |
| [9] | Xu Lei, Han Xiaoqiang, Zhang Jintao, Sun Haibiao. Hyaluronic acid around articular chondrocytes: production, transformation and function characteristics [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 768-773. |
| [10] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [11] | Zhang Jian, Lin Jianping, Zhou Gang, Fang Yehan, Wang Benchao, Wu Yongchang. Semi-quantitative MRI evaluation of cartilage degeneration in early knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 425-429. |
| [12] | Liu Shaohua, Zhou Guanming, Chen Xicong, Xiao Keming, Cai Jian, Liu Xiaofang. Changes in kinematic parameters after unicompartmental knee arthroplasty and high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 390-396. |
| [13] | Wang Chong, Zhang Meiying, Zhou Jian, Lao Kecheng. Early gait changes after total hip arthroplasty through direct anterior approach and posterolateral approach [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 359-364. |
| [14] | Sun Jinpeng, Liu Jun, Bai Yunfeng, Hua Feng, Wang Haoran, Zheng Hongrui, Wu Tao. Effects of suppressor of cytokine signaling 3 on osteogenic activity in the cartilage of adolescent idiopathic scoliosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(26): 4160-4165. |
| [15] | Wang Xianfeng, Ou Xin, Deng Biyong. Comparison of effects of exosomes secreted by different mesenchymal stem cells for the treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 3980-3985. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||