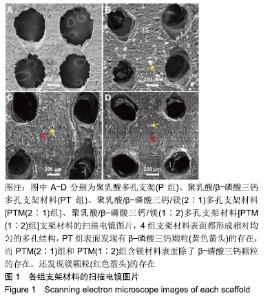
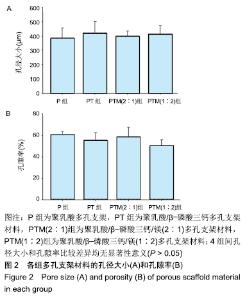
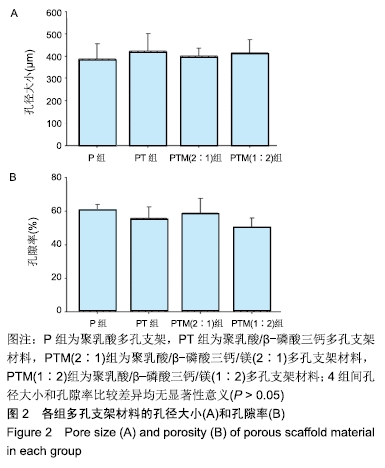
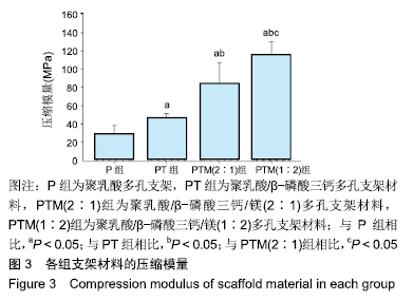
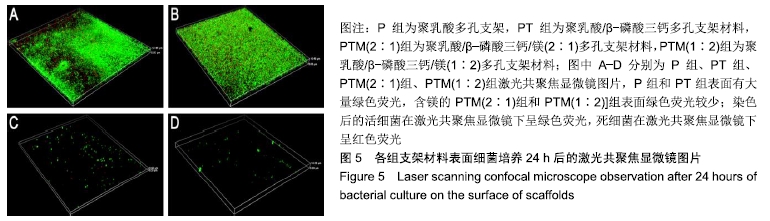
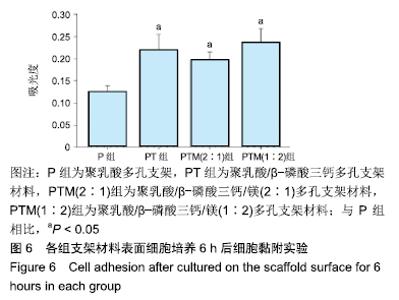
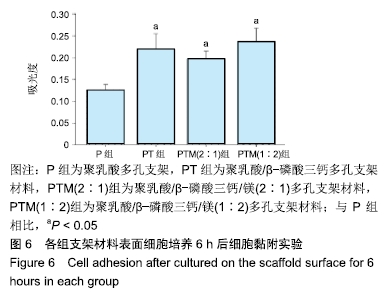
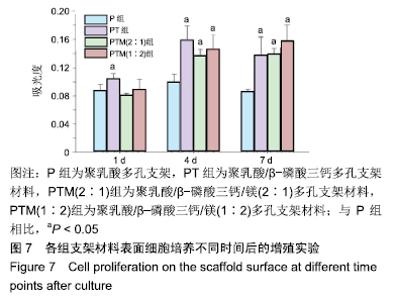
[1] CROES M, VAN DER WAL BCH, VOGELY HC .Impact of bacterial infections on osteogenesis: evidence from in vivo studies.J Orthop Res. 2019;37:2067-2076.
[2] ROMANO CL, SCARPONI S, GALLAZZI E, et al. Antibacterial coating of implants in orthopaedics and trauma: a classification proposal in an evolving panorama.J Orthop Surg Res.2015;10:157.
[3] LU C, HANSEN E, SAPOZHNIKOVA A, et al. Effect of age on vascularization during fracture repair.J Orthop Res.2008;26:1384-1389.
[4] DIMITRIOU R, JONES E, MCGONAGLE D, et al. Bone regeneration: current concepts and future directions. BMC Med.2011;9:66.
[5] LOU T, WANG X, SONG G, et al. Structure and properties of PLLA/β-TCP nanocomposite scaffolds for bone tissue engineering.J Mater Sci Mater Med.2015;26:5366.
[6] KIM HW, KNOWLES JC, KIM HE. Development of hydroxyapatite bone scaffold for controlled drug release via poly(epsilon-caprolactone) and hydroxyapatite hybrid coatings.J Biomed Mater Res B Appl Biomater. 2004;70(2):240-249.
[7] FEELEY BT, GALLO RA, SHERMAN S, et al. Management of osteoarthritis of the knee in the active patient.J Am Acad Orthop Surg. 2010;18:406-416.
[8] WITTE F. The history of biodegradable magnesium implants: a review. Acta Biomater.2010;6:1680-1692.
[9] YAZDIMAMAGHANI M, RAZAVI M, VASHAEE D, et al. Porous magnesium-based scaffolds for tissue engineering.Mat Sci Eng C. 2017;71:1253-1266.
[10] STAIGER MP, PIETAK AM, HUADMAI J, et al. Magnesium and its alloys as orthopedic biomaterials: a review.Biomaterials.2006;27:1728-1734.
[11] BROWN A, ZAKY S, RAY H JR, et al. Porous magnesium/PLGA composite scaffolds for enhanced bone regeneration following tooth extraction.Acta Biomater.2015;11:543-553.
[12] WAKSMAN R, PAKALA R, KUCHULAKANTI PK, et al. Safety and efficacy of bioabsorbable magnesium alloy stents in porcine coronary arteries.Catheter Cardio Inte.2006;68:607-617;discussion 618-609.
[13] ROBINSON DA, GRIFFITH RW, SHECHTMAN D, et al. In vitro antibacterial properties of magnesium metal against Escherichia coli,Pseudomonas aeruginosa and Staphylococcus aureus.Acta Biomater.2010;6:1869-1877.
[14] REN L, LIN X, TAN L, et al. Effect of surface coating on antibacterial behavior of magnesium based metals.Mater Lett.2011;65:3509-3511.
[15] CIFUENTES SC, FRUTOS E, GONZÁLEZ-CARRASCO JL, et al. Novel PLLA/magnesium composite for orthopedic applications: A proof of concept.Mater Lett.2012;74:239-242.
[16] HETRICK EM. Schoenfisch MH Reducing implant-related infections: active release strategies. Chem Soc Rev.2006;35:780-789.
[17] LI Y, LIU G, ZHAI Z, et al. Antibacterial properties of magnesium in vitro and in an in vivo model of implant-associated methicillin-resistant Staphylococcus aureus infection.Antimicrob Agents Chemother. 2014; 58(12):7586-7591.
[18] WALKER J, SHADANBAZ S, WOODFIELD TB, et al. Magnesium biomaterials for orthopedic application: A review from abiological perspective.J Biomed Mater Res B: Appl Biomater.2014;102B:1316-1331.
[19] LI X, LIU X, WU S, et al. Design of magnesium alloys with controllable degradationfor biomedical implants: From bulk to surface.Acta Biomater. 2016;45:2-30.
[20] LOCK J, WYATT E, UPADHYAYULA S, et al. Degradation and antibacterial properties of magnesium alloys in artificial urine for potential resorbable ureteral stent applications.J Biomed Mater Res A.2014;102(3):781-792.
[21] RAHIM MI, EIFLER R, RAIS B, et al. Alkalization is responsible for antibacterial effects of corrodingmagnesium.J Biomed Mater Res A. 2015;103:3526-3532.
[22] FOSS BL, GHIMIRE N, TANG R, et al.Bacteria and osteoblast adhesion to chitosan immobilized titanium surface: A race for the surface. Colloids Surf B Biointerfaces.2015;134:370-376.
[23] ARNETT T. Regulation of bone cell function by acid-base balance.Proc Nutr Soc. 2003;62(2):511-520.
|