Chinese Journal of Tissue Engineering Research ›› 2020, Vol. 24 ›› Issue (4): 524-531.doi: 10.3969/j.issn.2095-4344.1883
Previous Articles Next Articles
Fabrication and evaluation of biomimetic biodegradable tissue-engineered annulus fibrosus scaffold
Zhang Weihao1,2, Xu Baoshan2, Ma Xinlong2, Zhang Yang2, Guo Yue2, Du Lilong2, Xu Haiwei2, Zhang Kaihui1,2, Xia Jinjian1,2, Shao Pengfei1,2#br#
- 1Graduate School of Tianjin Medical University, Tianjin 300070, China; 2Department of Minimally Invasive Spine Surgery, Tianjin Hospital, Tianjin 300211, China
-
Received:2019-02-27Revised:2019-03-09Accepted:2019-06-04Online:2020-02-08Published:2019-12-31 -
Contact:Xu Baoshan, MD, Chief physician, Department of Minimally Invasive Spine Surgery, Tianjin Hospital, Tianjin 300211, China -
About author:Zhang Weihao, Master, Graduate School of Tianjin Medical University, Tianjin 300070, China; Department of Minimally Invasive Spine Surgery, Tianjin Hospital, Tianjin 300211, China -
Supported by:the National Natural Science Foundation of China (General Program), No. 31670983; the National Natural Science Foundation of China (Youth Program), No. 31500781; Applied Basic and Frontier Technology Research Project of Tianjin, No.15JCYBJC25300
CLC Number:
Cite this article
Zhang Weihao, Xu Baoshan, Ma Xinlong, Zhang Yang, Guo Yue, Du Lilong, Xu Haiwei, Zhang Kaihui, Xia Jinjian, Shao Pengfei. Fabrication and evaluation of biomimetic biodegradable tissue-engineered annulus fibrosus scaffold[J]. Chinese Journal of Tissue Engineering Research, 2020, 24(4): 524-531.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
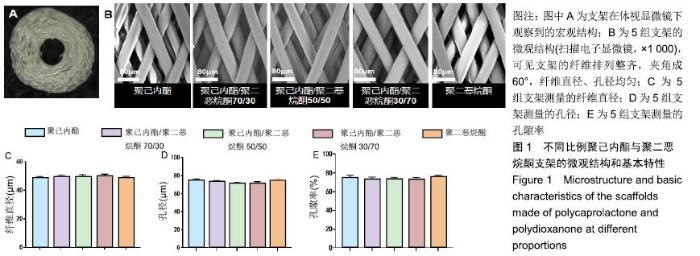
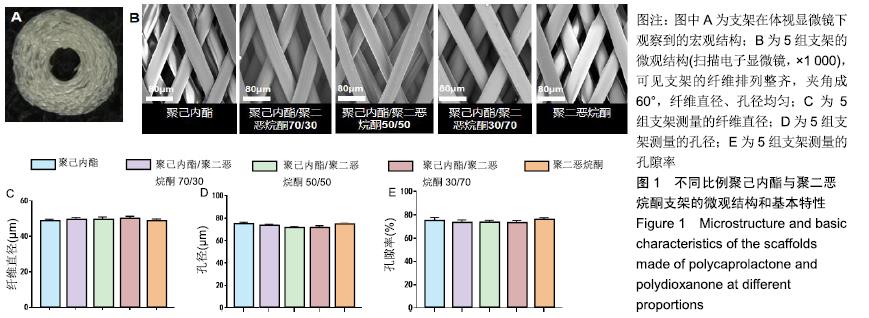
2.1 不同比例聚己内酯与聚二恶烷酮制备的支架的纤维形态 体式显微镜下可观察到支架宏观结构为圆柱形立体结构,由多层纤维均匀环绕而成,见图1A。扫描电镜下可见各层支架纤维呈多孔的菱形结构,夹角成60°,孔间叠合紧密,纤维丝分布均匀,见图1B。聚己内酯/聚二恶烷酮(70/30,50/50,30/70)混合支架纤维直径分别为(49.02± 1.74) μm,(49.53±1.49) μm,(50.41±1.06) μm,孔径分别为(73.05±1.43) μm,(72.49±1.35) μm,(71.92±1.37) μm,孔隙率为(73.56±2.32)%,(72.94±2.52)%,(73.53±1.59)%,聚二恶烷酮组支架纤维直径为(48.78±1.17) μm,孔径为(74.07±1.44) μm,孔隙率为(75.83±2.00)%;聚己内酯组支架纤维直径为(48.65±1.18) μm,孔径为(73.81±1.40) μm,孔隙率为(74.81±1.32)%。各组纤维基本参数差异无显著性意义(P > 0.05),见图1C-E。"
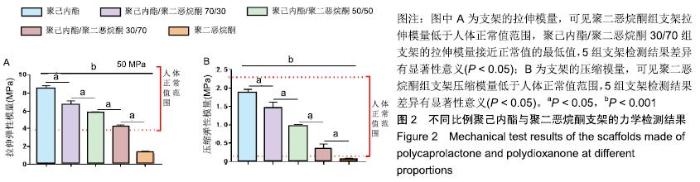
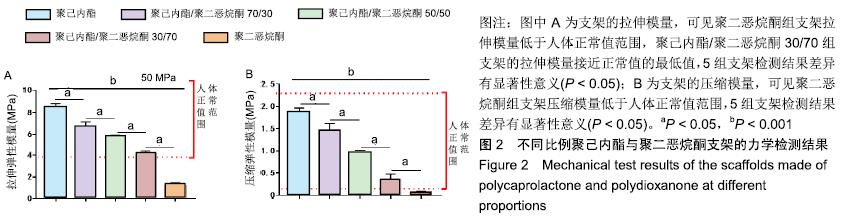
2.2 不同比例聚己内酯与聚二恶烷酮制备支架的力学性能检测 5组支架拉伸模量与压缩模量如图2所示。聚己内酯组支架的拉伸模量为(8.43±0.44) MPa,聚己内酯/聚二恶烷酮70/30组支架的拉伸模量为(6.67±0.48) MPa,聚己内酯/聚二恶烷酮50/50组支架的拉伸模量为(5.61± 0.31) MPa,聚己内酯/聚二恶烷酮30/70组支架的拉伸模量为(4.10±0.28) MPa,聚二恶烷酮组支架的拉伸模量(1.20±0.27) MPa;聚己内酯组支架的压缩模量为(1.88±0.08) MPa,聚己内酯/聚二恶烷酮70/30组支架的压缩模量为(1.46±0.15) MPa,聚己内酯/聚二恶烷酮50/50组支架的压缩模量为(0.92±0.09) MPa,聚己内酯/聚二恶烷酮30/70组支架的压缩模量为(0.35±0.13) MPa,聚二恶烷酮组支架的压缩模量(0.07±0.01) MPa。其中,聚二恶烷酮组的拉伸模量和压缩模量均低于人体纤维环力学阈 值[22-23],聚己内酯/聚二恶烷酮30/70组支架拉伸模量基本达到人体椎间盘所需拉伸模量的最低值,聚己内酯组的力学性能最好。"

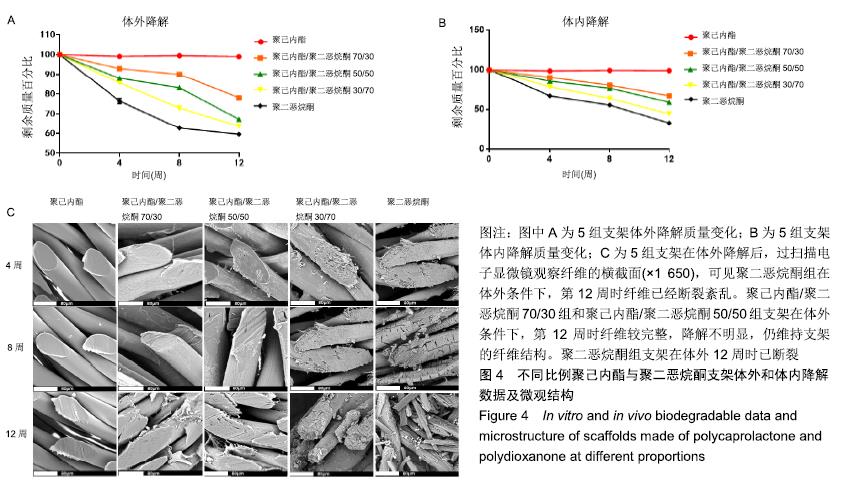
2.4 不同比例聚己内酯与聚二恶烷酮支架的体外降解与体内降解情况 在体内及体外降解过程中,各组支架降解趋势相似,但体内降解速度快于体外降解速度(P < 0.05),见图4A,B。其中聚二恶烷酮组支架体内降解速度最快。在第12周时体内植入的聚二恶烷酮支架已明显降解,这明显短于纤维环自我修复所需的时间,同时聚二恶烷酮组支架的力学性能不能达到人体纤维环力学阈值,所以将不再对聚二恶烷酮组支架进行后续实验。聚己内酯组支架无论在体内或体外都几乎没有降解。聚己内酯/聚二恶烷酮混合支架则随着聚二恶烷酮含量增加而使降解速度增加。随着时间的推移,混合支架中出现部分断丝或细小纤维从中剥离的现象,且聚二恶烷酮含量越多,现象越明显,见图4C。"
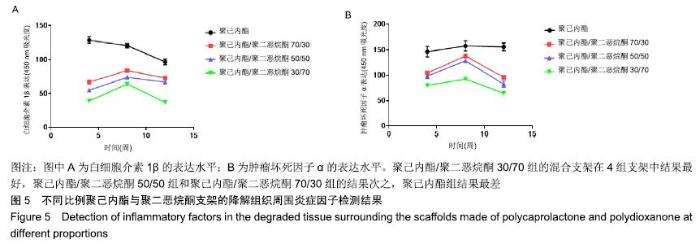
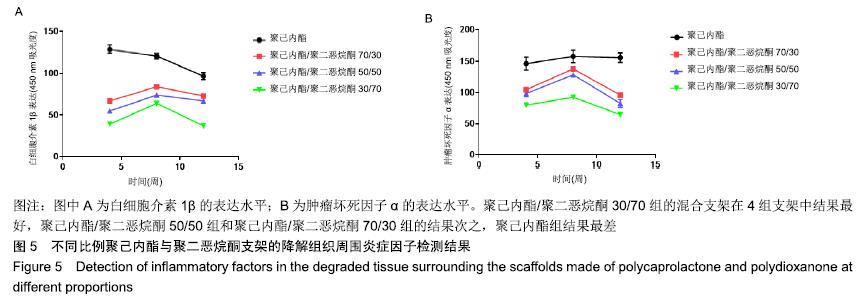
2.5 不同比例聚己内酯与聚二恶烷酮支架的降解组织周围炎症因子表达情况 ELISA检测结果显示,聚己内酯/聚二恶烷酮70/30组、聚己内酯/聚二恶烷酮50/50组、聚己内酯/聚二恶烷酮30/70组支架白细胞介素1β的表达先增高再降低,而在聚己内酯组中白细胞介素1β的表达则呈现持续降低的趋势。皮下埋植4周时,聚己内酯组的白细胞介素1β吸光度表现为最高,混合支架随着聚二恶烷酮含量的增加,表现为吸光度逐渐降低。8周时聚己内酯/聚二恶烷酮混合支架验的白细胞介素1β的表达水平均有升高,仍然表现为聚二恶烷酮含量越高的支架吸光度越低。12周时4组支架的白细胞介素1β的表达水平均为下降,且3组混合支架均低于聚己内酯组。聚己内酯组白细胞介素1β的表达水平在3个时间点(4,8,12周)中虽表现为逐渐下降,但在3个时间点白细胞介素1β的表达水平均高于聚己内酯/聚二恶烷酮混合支架组。肿瘤坏死因子α的表达水平和白细胞介素1β相似,见图5。"
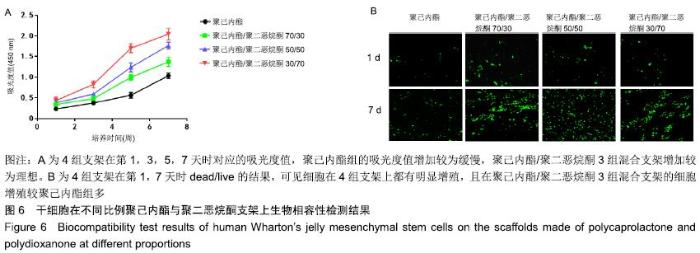
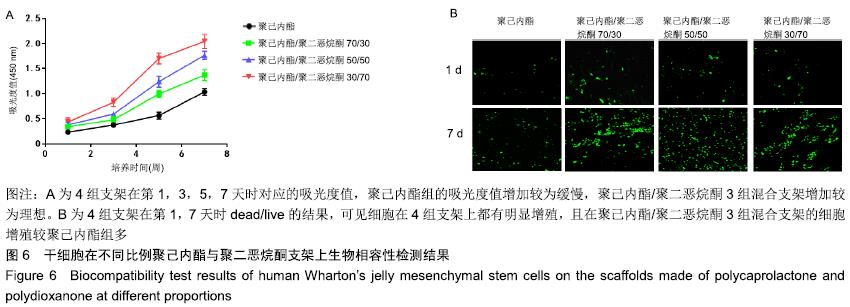
2.6 干细胞在不同比例聚己内酯与聚二恶烷酮纤维环支架上的生物相容性 2.6.1 CCK-8 结果显示,在培养第1天时,4组支架的培养液细胞数量没有明显差异;从第3天起,各组间差异有显著性意义(P < 0.05),聚二恶烷酮含量越高的混合支架细胞数量升高最明显。在第7天时,混合组支架的细胞数量明显高于聚己内酯组,且不同组的混合支架细胞数量差异有显著性意义(P < 0.05),可认为聚二恶烷酮对细胞增殖活性有促进作用,见图6A。 2.6.2 死活细胞检测 结果显示,4组支架上的细胞存活(绿色荧光)良好。第1天4组支架均有少量活细胞存在于视野中,但组间差异不明显。第7天时可观察到各组支架上活细胞数量明显增多,且聚己内酯/聚二恶烷酮混合支架活细胞数量较聚己内酯组多,见图6B。 "
| [1] MA K, CHEN S, LI Z, et al. Mechanisms of endogenous repair failure during intervertebral disc degeneration. Osteoarthritis Cartilage. 2018;27(1):41-48. [2] HAN I, ROPPER AE, KONYA D, et al. Biological approaches to treating intervertebral disk degeneration: devising stem cell therapies. Cell Transplant. 2015;24(11):2197-2208. [3] GU H, CHANG Y, ZENG S, et al. Wallis Interspinous Spacer for Treatment of Primary Lumbar Disc Herniation: Three-Year Results of a Randomized Controlled Trial. World Neurosurg. 2018;120:e1331-e1336. [4] AHLGREN BD, LUI W, HERKOWITZ HN, et al. Effect of anular repair on the healing strength of the intervertebral disc: a sheep model. Spine (Phila Pa 1976). 2000;25(17): 2165-2170. [5] BATEMAN AH, BALKOVEC C, AKENS MK, et al. Closure of the annulus fibrosus of the intervertebral disc using a novel suture application device-in vivo porcine and ex vivo biomechanical evaluation. Spine J. 2016;16(7):889-895. [6] SCHUTGENS EM, TRYFONIDOU MA, SMIT TH, et al. Biomaterials for intervertebral disc regeneration: past performance and possible future strategies. Eur Cell Mater. 2015;30:210-231. [7] RAJ PP. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008;8(1):18-44. [8] CHIANG CJ, CHENG CK, SUN JS, et al. The effect of a new anular repair after discectomy in intervertebral disc degeneration: an experimental study using a porcine spine model. Spine (Phila Pa 1976). 2011;36(10):761-769. [9] WANG F, SHI R, CAI F, et al. Stem Cell Approaches to Intervertebral Disc Regeneration: Obstacles from the Disc Microenvironment. Stem Cells Dev. 2015;24(21):2479-2495. [10] MAKHNI MC, CALDWELL JM, SAIFI C, et al. Tissue engineering advances in spine surgery. Regen Med. 2016; 11(2):211-222. [11] BOWLES RD, SETTON LA. Biomaterials for intervertebral disc regeneration and repair. Biomaterials. 2017;129:54-67. [12] ZHANG H, YU S, ZHAO X, et al. Stromal cell-derived factor-1α-encapsulated albumin/heparin nanoparticles for induced stem cell migration and intervertebral disc regeneration in vivo. Acta Biomater. 2018;72:217-227. [13] ISHIGURO H, KAITO T, YARIMITSU S, et al. Intervertebral disc regeneration with an adipose mesenchymal stem cell-derived tissue-engineered construct in a rat nucleotomy model. Acta Biomater. 2019;87:118-129. [14] ZHANG Y, TAO H, GU T, et al. The effects of human Wharton's jelly cell transplantation on the intervertebral disc in a canine disc degeneration model. Stem Cell Res Ther. 2015; 6:154. [15] ZHAO N, LV Z, MA J, et al. Fabrication of hydrophilic small diameter vascular foam scaffolds of poly(ε-caprolactone)/ polylactic blend by sodium hydroxide solution. Eur Polymer J. 2019;110:31-40 . [16] 李麒峰,徐宝山,杨强,等.聚己内酯/海藻酸钠/壳聚糖材料制备组织工程椎间盘双相支架[J].天津医药,2018,46(4):337-340. [17] CHOI DJ, CHOI SM, KANG HY, et al. Bioactive fish collagen/polycaprolactone composite nanofibrous scaffolds fabricated by electrospinning for 3D cell culture. J Biotechnol. 2015;205:47-58. [18] HAN CM, LIH E, CHOI SK, et al. Biodegradable sheath-core biphasic monofilament braided stent for bio-functional treatment of esophageal strictures. J Ind Eng Chem. 2018; 67:396-406. [19] GUPTA D, SHARMA U, CHAUHAN S, et al. Improved outcomes of scar revision with the use of polydioxanone suture in comparison to polyglactin 910: A randomized controlled trial. J Plast Reconstr Aesthet Surg. 2018;71(8): 1159-1163. [20] HOYER B, BERNHARDT A, HEINEMANN S, et al. Biomimetically mineralized salmon collagen scaffolds for application in bone tissue engineering. Biomacromolecules. 2012;13(4):1059-1066. [21] PAN Y, ZHOU X, WEI Y, et al. Small-diameter hybrid vascular grafts composed of polycaprolactone and polydioxanone fibers. Sci Rep. 2017;7(1):3615. [22] LONG RG, ROTMAN SG, HOM WW, et al. In vitro and biomechanical screening of polyethylene glycol and poly(trimethylene carbonate) block copolymers for annulus fibrosus repair. J Tissue Eng Regen Med. 2017;12(2): e727-e736. [23] BOWLES RD, SETTON LA. Biomaterials for intervertebral disc regeneration and repair. Biomaterials. 2017;129:54-67. [24] LI J, LIU C, GUO Q, et al. Regional variations in the cellular, biochemical, and biomechanical characteristics of rabbit annulus fibrosus. PLoS One. 2014;9(3):e91799. [25] YU J, SCHOLLUM ML, WADE KR, et al. ISSLS Prize Winner: A Detailed Examination of the Elastic Network Leads to a New Understanding of Annulus Fibrosus Organization. Spine (Phila Pa 1976). 2015;40(15):1149-1157. [26] PATTAPPA G, LI Z, PEROGLIO M, et al. Diversity of intervertebral disc cells: phenotype and function. J Anat. 2012; 221(6):480-496. [27] LI X, DOU Q, KONG Q. Repair and Regenerative Therapies of the Annulus Fibrosus of the Intervertebral Disc. J Coll Physicians Surg Pak. 2016;26(2):138-144. [28] IATRIDIS JC, NICOLL SB, MICHALEK AJ, et al. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013; 13(3):243-262. [29] HOOGENDOORN RJ, LU ZF, KROEZE RJ, et al. Adipose stem cells for intervertebral disc regeneration: current status and concepts for the future. J Cell Mol Med. 2008;12(6a): 2205-2216. [30] SATO M, KIKUCHI M, ISHIHARA M, et al. Tissue engineering of the intervertebral disc with cultured annulus fibrosus cells using atelocollagen honeycombshaped scaffold with a membrane seal (ACHMS scaffold). Med Biol Eng Comput. 2003;41(3):365-371. [31] VADALÀ G, RUSSO F, DI MARTINO A, et al. Intervertebral disc regeneration: from the degenerative cascade to molecular therapy and tissue engineering. J Tissue Eng Regen Med. 2013;9(6):679-690. [32] DU L, YANG Q, ZHANG J, et al. Engineering a biomimetic integrated scaffold for intervertebral disc replacement. Mater Sci Eng C Mater Biol Appl. 2018;96:522-529. [33] 李玉东,徐源,周强,等.聚乳酸-聚己内酯组织工程纤维环支架的制备及其性能研究[J].第三军医大学学报,2014,36(9):914-918. [34] BLAKENEY BA, TAMBRALLI A, ANDERSON JM, et al. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials. 2010;32(6):1583-1590. [35] THOMAS R, RAO A, CHAUHAN NS, et al. Melt spinning: A rapid and cost effective approach over ball milling for the production of nanostructured p-type Si 80 Ge 20 with enhanced thermoelectric properties. J Alloys Comp. 2019;781: 344-350.. [36] PADMAKUMAR S, PAUL-PRASANTH B, PAVITHRAN K, et al. Long-term drug delivery using implantable electrospun woven polymeric nanotextiles. Nanomedicine. 2018;15(1):274-284. [37] BENZ K, STIPPICH C, FISCHER L, et al. Intervertebral disc cell- and hydrogel-supported and spontaneous intervertebral disc repair in nucleotomized sheep. Eur Spine J. 2012;21(9): 1758-1768. |
| [1] | Gao Yan, Zhao Licong, Zhao Hongzeng, Zhu Yuanyuan, Li Jie, Sang Deen. Alteration of low frequency fluctuation amplitude at brain-resting state in patients with chronic discogenic low back pain [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1160-1165. |
| [2] | Liu Zhichao, Zhang Fan, Sun Qi, Kang Xiaole, Yuan Qiaomei, Liu Genzhe, Chen Jiang. Morphology and activity of human nucleus pulposus cells under different hydrostatic pressures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1172-1176. |
| [3] | Zou Gang, Xu Zhi, Liu Ziming, Li Yuwan, Yang Jibin, Jin Ying, Zhang Jun, Ge Zhen, Liu Yi. Human acellular amniotic membrane scaffold promotes ligament differentiation of human amniotic mesenchymal stem cells modified by Scleraxis in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1037-1044. |
| [4] | Yang Yang, Yao Yu, Shen Xiaotian, Liu Jiajia, Xue Jianhua. Expression and significance of interleukin-21 in intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 690-694. |
| [5] | Xu Yinqin, Shi Hongmei, Wang Guangyi. Effects of Tongbi prescription hot compress combined with acupuncture on mRNA expressions of apoptosis-related genes,Caspase-3 and Bcl-2, in degenerative intervertebral discs [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 713-718. |
| [6] | Li Wenjing, Li Haobo, Liu Congna, Cheng Dongmei, Chen Huizhen, Zhang Zhiyong. Comparison of different bioactive scaffolds in the treatment of regenerative pulp of young permanent teeth [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 499-503. |
| [7] | Zhou Jihui, Yao Meng, Wang Yansong, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Influence of novel nanoscaffolds on biological behaviors of neural stem cells and the related gene expression [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 532-536. |
| [8] | Ma Zhijie, Li Jingyu, Cao Fang, Liu Rong, Zhao Dewei. Influencing factors and biological property of novel biomedical materials: porous silicon carbide coated with bioactive tantalum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 558-563. |
| [9] | Liu Liu, Zhou Qingzhu, Gong Zhuo, Liu Boyan, Yang Bin, Zhao Xian. Characteristics and manufacturing techniques of collagen/inorganic materials for constructing tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 607-613. |
| [10] | Ye Haimin, Ding Linghua, Kong Weihao, Huang Zutai, Xiong Long. Role and mechanism of hierarchical microchanneled bone scaffolds in promoting osteogenesis and angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 621-625. |
| [11] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [12] | Liang Yan, Zhao Yongfei, Zhu Zhenqi, Liu Haiying, Mao Keya. Minimally invasive transforaminal lumbar interbody fusion in the treatment of sciatic scoliosis caused by lumbar disc herniation: a 2-year follow-up of coronal and sagittal balance [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 409-413. |
| [13] | Qian Xuankun, Huang Hefei, Wu Chengcong, Liu Keting, Ou Hua, Zhang Jinpeng, Ren Jing, Wan Jianshan. Computer-assisted navigation combined with minimally invasive transforaminal lumbar interbody fusion for lumbar spondylolisthesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3790-3795. |
| [14] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [15] | Liu Jun, Yang Long, Wang Weiyu, Zhou Yuhu, Wu Ying, Lu Tao, Shu Liping, Ma Minxian, Ye Chuan. Preparation and properties of poly3-hydroxybutyrate 4-hydroxybutyrate/polyethylene glycol/graphene oxide tissue-engineered scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3466-3472. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

