Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (35): 5703-5709.doi: 10.3969/j.issn.2095-4344.1489
Previous Articles Next Articles
Research progress of mechanism of protein glycosylation in the immune reaction under hypoxia
Chen Yao, Zhao Min, Li Wenhua
- (Department of Medicine, Xizang Minzu University, Xianyang 712082, Shaanxi Province, China)
-
Received:2019-04-13Online:2019-12-18Published:2019-12-18 -
Contact:Li Wenhua, Master, Professor, Department of Medicine, Xizang Minzu University, Xianyang 712082, Shaanxi Province, China -
About author:Chen Yao, Master candidate, Department of Medicine, Xizang Minzu University, Xianyang 712082, Shaanxi Province, China -
Supported by:the National Natural Science Foundation of China, No. 81760332 (to LWH); the Major Project of Natural Science Foundation of Science and Technology Department of Tibet, No. XZ2017ZRG-67(Z) (to LWH); the Natural Science Project of Science and Technology Department of Tibet, No. XZ-2018ZRG-71; the Young Talent Training Program of Xizang Minzu University, No. 18MDX02
CLC Number:
Cite this article
Chen Yao, Zhao Min, Li Wenhua. Research progress of mechanism of protein glycosylation in the immune reaction under hypoxia[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(35): 5703-5709.
share this article
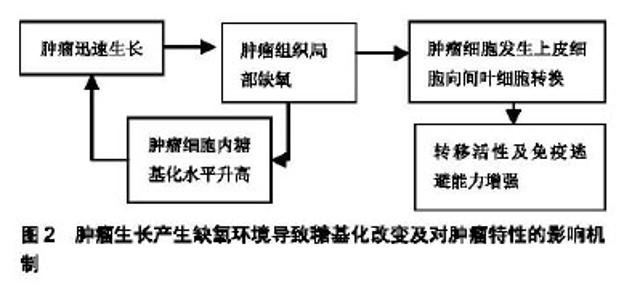
2.1 细菌所致的糖基化对免疫反应的影响 有研究表明,蛋白糖基化的合成受到细胞所处环境的影响[10-11]。Aon等[10]发现用实验室小规模的酵母培养与大规模的工业化酵母发酵过程相比,缺氧可导致酵母细胞的糖基化过程发生改变而影响了酵母细胞细胞壁的完整性。Gil-Marqués等[11]在鲍曼不动杆菌及绿脓杆菌在缺氧条件下感染上皮细胞及动物模型的实验中发现,缺氧可导致宿主细胞的抗菌作用增强并可减少病菌间的相互作用,但在缺氧的体内实验中却发现,缺氧可导致细菌的毒性增加。Cardlik等[12]在以编码有缺氧诱导因子1α的大肠杆菌喂养肠缺血大鼠的实验中发现,不管缺氧诱导因子1α基因是否表达,大鼠的肠缺血症状都有所改善。 有研究报道,致病菌为侵入宿主体内已演变出特异的糖基化程序。Barel等[13]通过实验发现,致病菌可通过糖基化与去糖基化模拟宿主细胞的聚糖结构,从而使病菌逃避宿主细胞的免疫识别。 Barel等[13]发现土拉热杆菌LVS可触发谷氨酰胺转运体SLC1A5导致单核细胞去糖基化而造成人单核细胞感染土拉热杆菌。在随后的实验中他们发现,土拉热杆菌侵入机体后可导致巨噬细胞中大量的糖苷酶及糖苷转移酶基因被激活,从而导致巨噬细胞中的N连接及O连接糖基化表达增加,最终造成了巨噬细胞感染。Barel等[14-15]研究发现土拉热杆菌感染后可对宿主细胞未折叠蛋白进行修饰并可对细胞自噬过程产生调节。 Belotserkovsky等[16]研究表明,非活化的CD4+T细胞表面的神经节糖苷与细菌细胞表面主要抗原脂多糖O-抗原聚糖的动态相互作用,促使了志贺氏菌与CD4+T细胞的粘连。从而表明聚糖之间的相互作用是导致志贺氏菌致病的关键。Sanchez等[17]报道,肠上皮细胞可分泌大量的黏液素糖蛋白,以形成固有的免疫屏障从而抵抗肠病毒的入侵。而肠道致病菌福氏志贺氏菌可通过分泌凝胶样黏液素对付这种黏液素屏障。这一过程可被细菌的毒力链触发,导致黏液素在细胞表面积累,从而在细胞表面形成凝胶样结构以支持病菌接近细胞并促发了随后的侵袭过程。 有研究表明,细菌特异的蛋白糖基化决定着其致病的毒力[18]。McDonald等[19]利用甲壳纲动物丰年虾的模型研究,表明5-N乙酰基-7-N-乙酰基-D丙胺酰-军团氨酸(Leg5Ac7AcAla,一种唾液酸的表达形式)可影响细菌外膜的完整,而其缺失可导致膜的渗透性增加,致使细菌对胆盐及杀菌肽的敏感性增加并可减弱细菌的毒力。有研究表明,存在于革兰阳性菌中富含丝氨酸重复序列(SRR)的糖蛋白是细菌细胞表面配基及毒力因子形成的关键[17,20]。有报道称,肺炎链球菌中富含丝氨酸重复序列的蛋白(PsrP)与致病域编码黏附素及肺炎链球菌致病有密切关系,先前的研究显示PsrP可通过位于基本区域273-341的氨基酸,对细菌附着于肺细胞表面的角蛋白10(K10)产生调节作用。 随后,在Sanchez等[17]的研究中,他们发现PsrP也可调节物种间的相互作用,进而促使大量病菌侵入鼻咽及肺而造成小鼠感染,并且PsrP可诱导病菌通过生物膜播散。在所有丝氨酸重复序列细菌细胞表面配基中了解最多的是来自猪链球菌的GspB,研究发现其有与唾液酸-T抗原碳水化合物装饰的血小板糖蛋白GPIbα黏附亲和的特性。Pybum等[20]所做的黏附区域晶体结构显示了在GspB黏附域(GspBBR)的3个子域中,有2个子域存在Ig-折叠。而包含Ig-折叠的蛋白通常存在于哺乳动物的免疫系统中,而且在免疫系统中它们通常有多种功能;然而,Ig-折叠蛋白却很少出现于病原体中,如果出现它们则表现为该病原体所特有的毒力因子。有研究表明,Ig-折叠蛋白包含于多种毒力因素中,表现为其是菌毛的组成成分(碳水化合物粘合的菌毛)且存在于细菌细胞表面配基中的多种宿主特异性受体中,其中包括识别黏附基质分子的细菌表面成分(MSCRAMMs)及侵袭素[20]。 Salah Ud-Din等[18]研究发现,多数致病菌的鞭毛可促使该病原菌侵入宿主体内,并使其能够躲避免疫监视而长期的存在与宿主体内。他们通过对幽门螺旋杆菌及空肠弯曲杆菌的研究发现,带有特异的糖假氨基酸鞭毛蛋白的糖基化在功能性鞭毛的生物合成中扮演着重要角色。因此,他们推出鞭毛蛋白的糖基化在细菌的运动及致病中都起着重要的作用。Garfoot等[21]发现,蛋白的O-甘露糖基化可增加组织浆菌酵母细胞的耐热性从而促使其更易侵入哺乳动物的免疫细胞。Bloch等[22]发现口腔致病菌福赛斯坦纳菌拥有一个独特的携带有大量O-聚糖的表层,其在诱发上皮组织感染中起着重要的作用。 高原环境的特征包括氧含量低、气压低、辐射强及寒冷。作为首要的环境因素缺氧是机体发生损伤的基础,是导致一系列疾病发生的关键[23],并有研究证明,缺氧会导致缺氧诱导因子1α的蓄积[4,8]。在高原环境中蛋白糖基化是否参与疾病的形成还有待进一步研究。 2.2 病毒所致糖基化对免疫反应的影响 Tolnay等[24]发现病毒感染,其中包括H5N1高致病性禽流感在内的病毒都会诱导缺氧诱导因子1的表达。而Peixoto等[8]研究显示缺氧及作为缺氧诱导因子的表达,可通过促使糖基转移酶的不平衡表达而干扰细胞表面的分子糖基化过程。有研究表明,蛋白的糖基化修饰在病毒的传染、病毒颗粒的形成及其发生免疫逃避的过程中都扮演着重要角色。Bagdonaite等[25]发现,个别糖基化位点在病毒的生物学活性方面扮演着重要角色。而且大量分布于所有糖蛋白表面的聚糖对病毒表位的掩盖直接影响了机体的免疫识别。 Hargett等[26]发现人免疫缺陷病毒Ⅰ型(human immunodeficiency virus1,HIV-1)胞膜N-糖基化位点N262,N488以及N301的糖基化对于其躲避宿主的免疫监视有重要作用。有研究报道,一般地在广泛中和性抗体的演进中,HIV-1病毒的初始变异通常发生于HIV-1胞膜V1、V2或V3区域的胞膜基因中,而这些表位通常可被广泛中和抗体识别。而在Ji等[27]对于急性CRFO1_AE HIV-1感染患者的研究中发现,病毒胞膜基因V4区域的糖基化突变,至今还没有发现有广泛的中和性抗体产生。然而,V4区域的不断演进其中包括点突变、缺失、插入都会通过促使中和性特异抗体的演进并导致病毒发生免疫逃避。另有研究报道,HIV-1免疫逃避的发生主要与不稳定的HIV-1逆转录酶有关[26,28]。 研究发现,HBsAg的变异体在Q129N或T131N/ M133T发生的N-糖基化对HBsAg变异体的抗原性及免疫原性都产生了深远的影响[29]。 Sun等[30]报道,HA蛋白上存在着大量的糖基化位点,其糖基化位点的变异是导致季节性人流感爆发的根本原因。Mohebbi等[31]通过系统分析A/H1N1pdm09的HA基因显示该病毒是6B1的分支,其特征是在S84N,S162N及1216T位点发生了氨基酸的改变,其中在162位点发生的糖基化导致了病毒抗原性的改变而导致了最终的流行。Huang等[32]在对H3N2流感病毒的研究中发现在血凝素1(HA1)上A位点的N-糖基化突变是最常见的,而其B位点上的糖基化变异是最易诱发机体发生免疫应答的。Koel等[29]研究发现,在A/H3N2型流感病毒血凝集素基因的A,B,C,E及L表位中对病毒的抗原性起决定作用的是与RBD相邻的氨基酸,而这些血凝集素的基因位点除了145位于表位A外,其余的如:155,156,158,159,189及193均位于B表位。Zhao等[33]在对H5N1流感病毒的研究中发现在HA蛋白158位点的变异会导致HA蛋白158-160位点发生N-糖基化,而这些位点的N-糖基化会导致病毒的侵袭力增加,并通过诱导机体黏液素相关基因的表达,激活钙激活的氯通道ClCa3调节黏液的产生与分泌,而诱发宿主发生更加强烈的免疫应答和炎症反应。Han等[34]利用CRISPR/Cas9敲除在人肺上皮细胞中表达的禽流感H5N1病毒后发现,侵入人体后的H5N1中有很多基因涉及唾液酸的生物合成及相关糖基化通路的激活。其中,包括SLC35A1(一种唾液酸的转运体)是导致流感A病毒受体表达及侵入人体的关键。有研究发现,氨基酸的替换及糖基化改变(T127N,L150S,T188N及D189N糖基化位点的增加)是导致H9N2免疫原性发生改变并导致其发生免疫逃避的重要因素[35]。Sun等[30]研究发现,流感病毒蛋白的糖基化可掩盖病毒的免疫识别位点而使其逃避机体的免疫监视。有研究发现,NKp46与NKp44和血凝素蛋白的相互作用在自然杀伤细胞调节的病毒清除中发挥了重要作用[36]。并且,有研究显示流感病毒感染可扰乱宿主的黏膜免疫系统[36]。 用染色荧光免疫检验和蛋白质印迹技术共同检测在Huh7细胞中变体乙肝表面抗原的表达时发现,变体乙肝表面抗原的Q129N及T131N/M133T出现了新的N-糖基化,并由此而改变了变体乙肝表面抗原的抗原性及免疫原性[37]。 Fedechkin等[38]研究发现,呼吸系统多核体病毒(RSV)胞膜的G糖蛋白可通过它与宿主细胞的贴附及对宿主免疫的调节而促使机体发病。尽管G糖蛋白是呼吸道多核体病毒-中和抗体的靶标,然而把它作为抗原形成的疫苗却被它产生的各种各样的糖基化阻挡在核心区域外而无法发挥作用。Leemans等[39]通过对呼吸道多核体病毒融合蛋白研究发现,该蛋白包含5个N-糖基化位点(F2亚基的N27及N70,F1亚基上的N500及p27的N116及N126),其中位于p27中的N116 N-聚糖缺失可导致以F DNA免疫构建的BALB/c小鼠的抗体反应增强。 Wen等[40]研究发现Zika病毒可利用病毒胞膜糖基化改变抵御宿主的免疫反应。Bagdonaite等[25]强调,蛋白的糖基化对于所有蛋白结构的形成、功能的行驶及免疫识别都起着重要作用。同样在高原环境中机体也存在缺氧及缺氧诱导因子1的表达,在这种条件下是否会导 致糖基化的改变还未见相关报道。病毒感染形成缺氧环境导致糖基化改变对机体免疫影响的总结见表1。 2.3 肿瘤所制糖基化对免疫反应的影响 大量研究表明,缺氧可导致蛋白糖基化发生显著的改变[4]。而缺氧又是大部分实体肿瘤的发展常态[37,41]。Hanover等[42]发现在许多肿瘤细胞中基态O连接N乙酰葡萄糖胺酶的升高会导致肿瘤细胞优先生长,并通过提升氨基已醣(HBP)通路的活性增加O-N-乙酰葡糖胺糖基化修饰及协调改变细胞表面的N-和O-连接聚糖或导致肿瘤细胞发生上皮细胞至间叶细胞的转换[43-45],而增加了肿瘤的转移活性及免疫逃避能力[46]。Burchell等[47]发现黏液素型O-连接糖基化存在于90%的乳腺癌患者中,通过观察发现其可增加蛋白的唾液酸基化表达,并导致聚糖侧链改变。而黏液素型O-连接糖基化会影响肿瘤细胞的生长及发展。 Albuquerque等[48]在对MDA-MB-231系三阴乳腺癌细胞的缺血缺氧实验中发现,在缺氧条件下过表达的缺氧诱导因子1α可导致肿瘤细胞发生从上皮细胞到间叶细胞的转换,并显著的上调了岩藻糖基转移酶和唾液酸基转移酶在肿瘤细胞中的表达。Rego等[49]发现炎性乳癌及粉刺型导管内癌与其他型乳腺癌的预后相比时发现它们的预后较差,而在此过程中蛋白质糖基化也发挥了重要作用。肿瘤生长产生缺氧环境导致糖基化改变及对肿瘤特性的影响机制见图2。 "
| [1]Greville G, McCann A, Rudd PM, et al. Epigenetic regulation of glycosylation and the impact on chemo-resistance in breast and ovarian cancer. Epigenetics. 2016;11(12):845-857.[2]Lan Y, Hao C, Zeng X, et al. Serum glycoprotein-derived N- and O-linked glycans as cancer biomarkers. Am J Cancer Res. 2016;6(11):2390-2415.[3]Josic D, Martinovic T, Pavelic K. Glycosylation and metastases. Electrophoresis. 2019;40(1):140-150.[4]Silva-filho AF, Sena WLB, Lima LRA, et al. Glycobiology Modifications in Intratumoral Hypoxia: The Breathless Side of Glycans Interaction. Cell Physiol Biochem. 2017;41(5): 1801-1829.[5]Christiansen MN,Chik J,Lee L,et al.Cell surface protein glycosylation in cancer. Proteomics. 2014;14(4-5):525-646.[6]Blondeel EJM, Aucoin MG. Supplementing glycosylation: A review of applying nucleotide-sugar precursors to growth medium to affect therapeuticrecombinant protein glycoform distributions. Biotechnol Adv. 2018;36(5):1505-1523.[7]Tan Z,Wang C,Li X,et al.Bisecting N-Acetylglucosamine Structures Inhibit Hypoxia-Induced Epithelial-Mesenchymal Transition in Breast Cancer Cells. Front Physiol. 2018;9:210.[8]Peixoto A, Fernandes E, Gaiteiro C, et al.Hypoxia enhances the malignant nature of bladder cancer cells and concomitantly antagonizes protein O-glycosylation extension. Oncotarget. 2016;7(39):63138-63157.[9]Veillon L, Zhou S, Mechref Y. Quantitative Glycomics: A Combined Analytical and Bioinformatics Approach. Methods Enzymol. 2017;585:431-477.[10]Aon JC, Sun J, Leighton JM, et al. Hypoxia-elicited impairment of cell wall integrity, glycosylation precursor synthesis, and growth in scaled-up high-cell density fed-batch cultures of Saccharomyces cerevisiae. Microb Cell Fact. 2016; 15(1):142. [11]Gil-Marqués ML,Pachón-Ibáñez ME,Pachón J,et al.Effect of Hypoxia on the Pathogenesis ofAcinetobacter baumannii and Pseudomonas aeruginosa In Vitro and in Murine Experimental Models of Infection. Infect Immun. 2018;86(10). pii: e00543-18.[12]Gardlik R,Hodosy J,Palffy R,et al.Effects of Orally Administered Bacteria Carrying HIF-1α Gene in an Experimental Model of Intestinal Ischemia. Arch Med Res. 2010l;41(5):332-337.[13]Barel M,Charbit A.Role of Glycosylation/Deglycolysation Processes in Francisella tularensis Pathogenesis. Front Cell Infect Microbiol. 2017;7:71.[14]Barel M,Harduin-Lepers A, Portier L, et al. Host glycosylation pathways and the unfolded protein response contribute to the infection by Francisella. Cellular Microbiology. 2016;12(18): 1763-1781.[15]Miller C, Celli J. Avoidance and subversion of eukaryotic homeostatic autophagy mechanisms by bacterial pathogens. Mol Biol. 2016;17(428):3387-3398.[16]Belotserkovsky I,Brunner K, Pinaud L, et al. Glycan-Glycan Interaction Determines Shigella Tropism toward Human T Lymphocytes. mBio. 2018,9(1):e2309.[17]Sanchez C, Shivshankar P, Stol K, et al. The Pneumococcal Serine-Rich Repeat Protein Is an Intra-Species Bacterial Adhesin That Promotes Bacterial Aggregation In Vivo and in Biofilms. PLoS Pathog. 2010;6(8):e1001044.[18]Salah Ud-Din AIM,Roujeinikova A.Flagellin glycosylation with pseudaminic acid in Campylobacter and Helicobacter: prospects for development of novel therapeutics. Cell Mol Life Sci. 2018;75(7):1163-1178.[19]McDonald ND,DeMeester KE, Lewis A L, et al. Structural and functional characterization of a modified legionaminic acid involved in glycosylation of a bacterial lipopolysaccharide. J Biol Chem. 2018;293(49):19113-19126. [20]Pyburn T, Bensing B, Xiong Y, et al. A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate Receptors. PLoS Pathog.2011l;7(7): e1002112.[21]Garfoot AL, Goughenour KD, Wüthrich M, et al. O-Mannosylation of Proteins Enables Histoplasma Yeast Survival at Mammalian Body Temperatures.2018;9(1): e2121.[22]Bloch S, Zwicker S, Bostanci N, et al. Immune response profiling of primary monocytes and oral keratinocytes to differentTannerella forsythia strains and their cell surface mutants.Molecular Oral Microbiology. 2018;33(2):155-167.[23]Luo B,Li J,Yang T, et al. Evaluation of renal excretion and pharmacokinetics of furosemide in rats after acute exposure to high altitude at 4300 m. Biopharm Drug Dispos.2018;39(8): 378-387.[24]Tolnay AE,Baskin CR,Tumpey TM,et al.Extrapulmonary tissue responses in cynomolgus macaques (Macaca fascicularis) infected with highly pathogenic avian influenza A (H5N1) virus. Arch Virol.2010;155(6):905-914.[25]Bagdonaite I, Wandall HH. Global Aspects of Viral Glycosylation. Glycobiology. 2018;7(28):443-467.[26]Hargett AA, Wei Q, Knoppova B, et al. Defining HIV-1 Envelope N-glycan Microdomains Through Site-specific Heterogeneity Profiles. J Virol. 2018;93(1). pii: e01177-1118.[27]Ji Y, Han X, Tian W, et al. V4 region of the HIV-1 envelope gene mediates immune escape and may not promote the development of broadly neutralizing antibodies. Vaccine. 2018;36(50):7700-7707.[28]Roberts JD, Bebenek K, Kunkel TA.The accuracy of reverse transcriptase from HIV-1.Science.1988;242(4882): 1171-1173.[29]Koel BF, Burke DF, Bestebroer TM, et al. Substitutions Near the Receptor Binding Site Determine Major Antigenic Change During Influenza Virus Evolution. Science. 2013;342(6161): 976-979.[30]Sun S, Wang Q, Zhao F, et al. Glycosylation site alteration in the evolution of influenza A (H1N1) viruses. PLoS One. 2011; 6(7):e22844.[31]Mohebbi A, Fotouhi F, Jamali A, et al. Molecular Epidemiology of the Hemagglutinin gene of prevalent influenza virus A/H1N1/ pdm09 among patient in Iran. Virus Res. 2019;259:38-45. [32]Huang Z, Yu L, Huang P, et al. Charged amino acid variability related to N-glyco -sylation and epitopes in A/H3N2 influenza: Hem -agglutinin and neuraminidase. PLOS ONE. 2017;12(7): e178231.[33]Zhao D, Liang L, Wang S, et al. Glycosylation of the Hemagglutinin Protein of H5N1 Influenza Virus Increases Its Virulence in Mice by Exacerbating the Host Immune Response. J Virol. 2017;91(7). pii: e02215-2216. [34]Han J, Perez J T, Chen C, et al. Genome-wide CRISPR/Cas9 Screen Identifies Host Factors Essential for Influenza Virus Replication. Cell Reports. 2018;23(2):596-607.[35]Peacock TP, Harvey WT, Sadeyen J, et al. The molecular basis of antigenic variation among A(H9N2) avian influenza viruses. Emerg Microbes Infect. 2018;7(1):176. [36]Zhao D, Liang L, Wang S, et al. Glycosylation of the Hemagglutinin Protein of H5N1 Influenza Virus Increases Its Virulence in Mice by Exacerbating the Host Immune Response. J Virol. 2017;91(7). pii: e02215-16. [37]Chouaib S,Messai Y, Couve S, et al. Hypoxia Promotes Tumor Growth in Linking Angiogenesis to Immune Escape. Front Immunol. 2012;3:21.[38]Fedechkin SO, George NL, Wolff JT, et al. Structures of respiratory syncytial virus G antigen bound to broadly neutralizing antibodies. Sci Immunol. 2018;3(21). pii: eaar3534. [39]Leemans A, Boeren M, Van der Gucht W, et al. Removal of the N-Glycosylation Sequon at Position N116 Located in p27 of the Respiratory Syncytial Virus Fusion Protein Elicits Enhanced Antibody Responses after DNA Immunization. Viruses. 2018;10(8). pii: E426[40]Wen D,Li S,Dong F,et al.N-glycosylation of Viral E Protein Is the Determinant for Vector Midgut Invasion by Flaviviruses. MBio. 2018;9(1). pii: e00046-18.[41]Tang C,Yu J.Hypoxia-inducible factor-1 as a therapeutic target in cancer. J Gastroenterol Hepatol. 2013;28(3): 401-405.[42]Hanover JA,Chen W,Bond MR.O-GlcNAc in cancer: An Oncometabolism-fueled vicious cycle. 2018;50(3):155-173.[43]Vajaria BN, Patel PS. Glycosylation: a hallmark of cancer?. Glycoconj J. 2017;34(2):147-156.[44]Carvalho-cruz P,Alisson-Silva F,Todeschini AR, et al. Cellular glycosylation senses metabolic changes and modulates cell plasticity during epithelial to mesenchymal transition. Dev Dyn. 2018;247(3):481-491.[45]Niu Y, Xia Y, Wang J, et al. O-GlcNAcylation promotes migration and invasion in human ovarian cancer cells via the RhoA/ROCK/MLC pathway. Mol Med Rep.2017;15(4): 2083-2089.[46]Hanover JA, Chen W, Bond MR. O-GlcNAc in cancer: An Oncometabolism-fueled vicious cycle. J Bioenerg Biomembr. 2018;50(3):155-173.[47]Burchell JM, Beatson R, Graham R, et al. O-linked mucin-type glycosylation in breast cancer. Biochem Soc Trans. 2018;46(4):779-788. [48]Albuquerque APB, Balmaña M, Mereiter S, et al. Hypoxia and serum deprivation induces glycan alterations in triple negative breast cancer cells. Biol Chem. 2018;399(7):661-672.[49]Rêgo MJ, Vieira De Mello GS, Da Silva Santos CA, et al. Implications on glycobiological aspects of tumor hypoxia in breast ductal carcinoma in situ. Med Mol Morphol. 2013;46(2): 92-96.[50]Chugh S,Barkeer S,Rachagani S,et al.Disruption of C1galt1 Gene Promotes Development and Metastasis of Pancreatic Adenocarcinomas in Mice. Gastroenterology.2018;155(5): 1608-1624.[51]Coelho R, Marcos-Silva L, Mendes N, et al. Mucins and Truncated O-Glycans Unveil Phenotypic Discrepancies between Serous Ovarian Cancer Cell Lines and Primary Tumours. Int J Mol Sci. 2018;19(7). pii: E2045..[52]Höti N, Yang S, Hu Y, et al. Overexpression of α (1,6) fucosyltransferase in the development of castration-resistant prostate cancer cells. Prostate Cancer Prostatic Dis. 2018; 21(1):137-146. [53]Khan MI, Rath S, Adhami VM, et al. Hypoxia Driven Glycation: Mechanisms and Therapeutic Opportunities. 2017;49:75-82.[54]Zhang J,Shao S,Han D, et al. High mobility group box 1 promotes the epithelial-to-mesenchymal transition in prostate cancer PC3 cells via the RAGE/NF-κB signaling pathway. Int J Oncol. 2018;53(2):659-671.[55]Zhang J,Zhang Q,Lou Y,et al.Hypoxia-inducible factor-1α/interleukin-1β signaling enhances hepatoma epithelial-mesenchymal transition through macrophages in a hypoxic-inflammatory microenvironment. Hepatology. 2018; 67(5):1872-1889.[56]Yamada N, Kato-Kogoe N, Yamanegi K, et al.Inhibition of Asparagine-linked Glycosylation Participates in Hypoxia-induced Down-regulation of Cell-surface MICA Expression. Anticancer Res. 2018;38(3):1353-1359.[57]Tan Z,Wang C,Li X,et al.Bisecting N-Acetylglucosamine Structures Inhibit Hypoxia-Induced Epithelial-Mesenchymal Transition in Breast Cancer Cells. Front Physiol. 2018;9:210.[58]Jones RB, Dorsett KA, Hjelmeland AB, et al. The ST6Gal-I sialyltransferase protects tumor cells against hypoxia by enhancing HIF-1α signaling. J Biol Chem. 2018;293(15): 5659-5667.[59]Dos SS, Sheldon H, Pereira JX, et al. Galectin-3 acts as an angiogenic switch to induce tumor angiogenesis via Jagged-1/Notch activation. Oncotarget. 2017;8(30): 49484-49501.[60]Ferrer CM, Lynch TP, Sodi VL, et al.O-GlcNAcylation Regulates Cancer Metabolism and Survival Stress Signaling via Regulation of the HIF-1 Pathway. Molecular Cell. 2014; 54(5):820-831.[61]Yoshida Y,Furukawa J,Naito S, et al.Quantitative analysis of total serum glycome in human and mouse. Proteomics. 2016; 16(21):2747-2758. |
| [1] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [2] | Jiang Yong, Luo Yi, Ding Yongli, Zhou Yong, Min Li, Tang Fan, Zhang Wenli, Duan Hong, Tu Chongqi. Von Mises stress on the influence of pelvic stability by precise sacral resection and clinical validation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1318-1323. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Gu Xia, Zhao Min, Wang Pingyi, Li Yimei, Li Wenhua. Relationship between hypoxia inducible factor 1 alpha and hypoxia signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1284-1289. |
| [5] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [6] | Liu Cong, Liu Su. Molecular mechanism of miR-17-5p regulation of hypoxia inducible factor-1α mediated adipocyte differentiation and angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1069-1074. |
| [7] | Zhao Min, Feng Liuxiang, Chen Yao, Gu Xia, Wang Pingyi, Li Yimei, Li Wenhua. Exosomes as a disease marker under hypoxic conditions [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1104-1108. |
| [8] | Jiao Hui, Zhang Yining, Song Yuqing, Lin Yu, Wang Xiuli. Advances in research and application of breast cancer organoids [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1122-1128. |
| [9] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [10] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [11] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [12] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [13] | Chen Junyi, Wang Ning, Peng Chengfei, Zhu Lunjing, Duan Jiangtao, Wang Ye, Bei Chaoyong. Decalcified bone matrix and lentivirus-mediated silencing of P75 neurotrophin receptor transfected bone marrow mesenchymal stem cells to construct tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 510-515. |
| [14] | Li Xingping, Xiao Dongqin, Zhao Qiao, Chen Shuo, Bai Yiguang, Liu Kang, Feng Gang, Duan Ke. Preparation and properties of copper-loaded antibacterial functional film on titanium surface [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 553-557. |
| [15] | Shi Xiaoxiu, Mao Shilong, Liu Yang, Ma Xingshuang, Luo Yanfeng. Comparison of tantalum and titanium (alloy) as orthopedic materials: physical and chemical indexes, antibacterial and osteogenic ability [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 593-599. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

