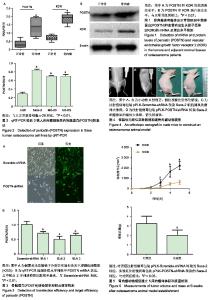
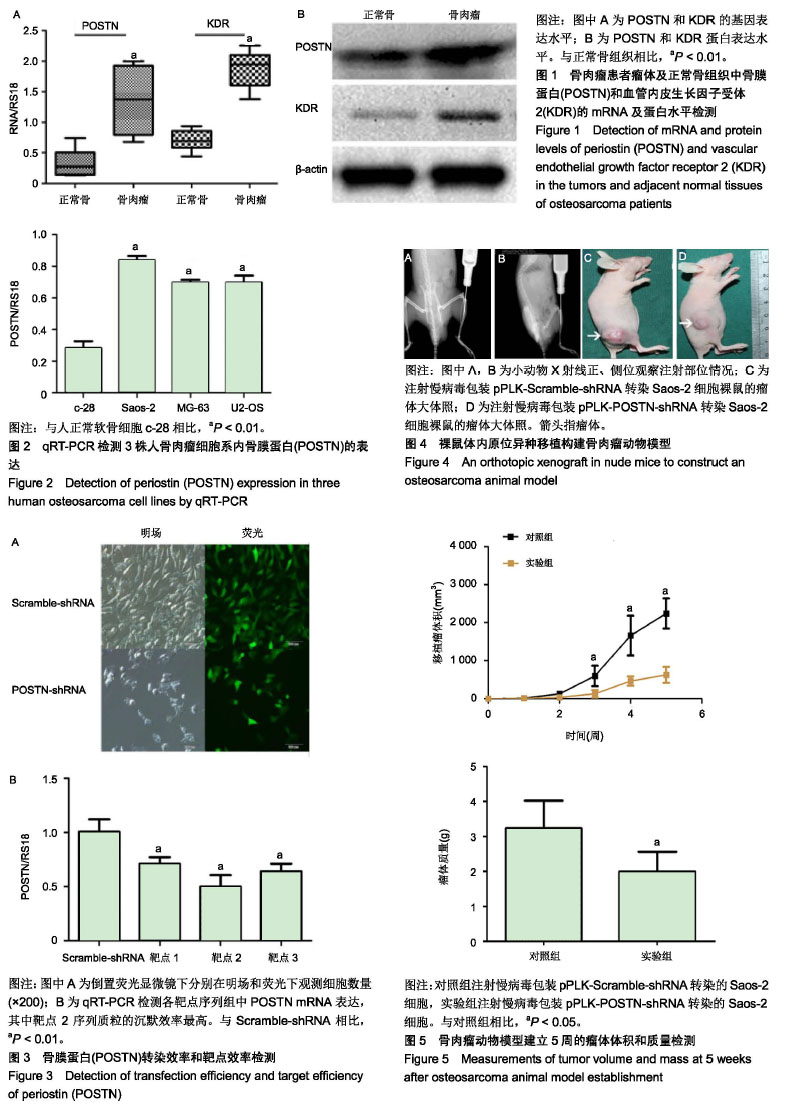
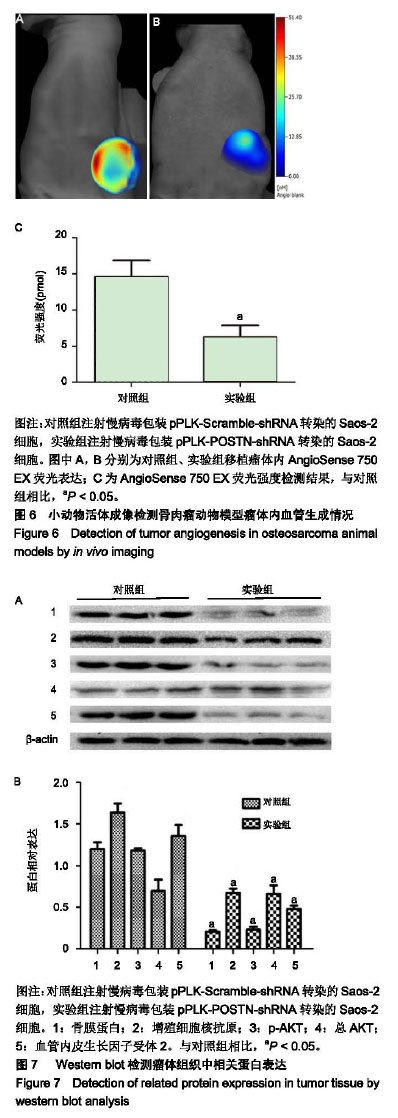
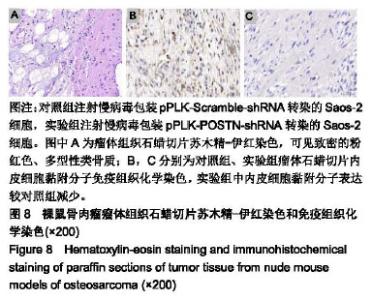
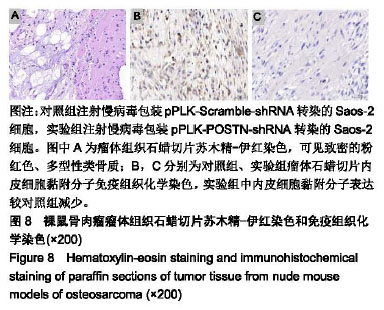
| [1]Ottaviani G,Jaffe N.The epidemiology of osteosarcoma.Cancer Treat Res.2009;152:3.[2]Bacci G,Picci P,Ferrari S,et al.Primary chemotherapy and delayed surgery for nonmetastatic osteosarcoma of the extremities. Results in 164 patients preoperatively treated with high doses of methotrexate followed by cisplatin and doxorubicin.Cancer.2015; 72(11):3227-3238.[3]Peng L,Suzanne O,Wenguang F,et al.Hypoxia-responsive growth factors upregulate periostin and osteopontin expression via distinct signaling pathways in rat pulmonary arterial smooth muscle cells. J Appl Physiol. 2004;97(4):1550-1558.[4]Bao S,Ouyang G,Bai X,et al.Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway.Cancer Cell.2004;5(4):329-339.[5]Hu F, Wang W, Zhou HC, et al. High expression of periostin is dramatically associated with metastatic potential and poor prognosis of patients with osteosarcoma. World J Surg Oncol.2014;12(1):287.[6]项锋钢,马桂馨.肺癌组织中血管内皮生长因子受体的表达及其与转移和预后的关系[J].中国组织工程研究与临床康复,2005,9(6):206-208.[7]Laurent L,Fabrice LB,Jacques H.Endothelial cell migration during angiogenesis. CircRes.2007;100(6):782-794.[8]Thuwajit C,Utispan K,Sonongbua J,et al.Abstract 419: Periostin activates integrin alpha5/beta1 through AKT-dependent pathway in invasion of cholangiocarcinoma.Cancer Res.2011; 71(8 Supplement):419.[9]Angelini A, Ceci F, Castellucci P, et al.The role of 18 F-FDG PET/CT in the detection of osteosarcoma recurrence.Eur J Nucl Med Mol Imaging. 2017;44(10):1712-1720. [10]Stefano F,Emanuela P.Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr Opin Oncol. 2007; 19(4): 341-346.[11]Mirabello L,Troisi RJ,Savage SA.Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program.Cancer.2010;115(7): 1531-1543.[12]Saaristo A,Karpanen TK. Mechanisms of angiogenesis and their use in the inhibition of tumor growth and metastasis.Oncogene. 2000;19(53): 6122-6129.[13]Mei J,Gao Y,Zhang L,et al.VEGF-siRNA silencing induces apoptosis, inhibits proliferation and suppresses vasculogenic mimicry in osteosarcoma in vitro.Exp Oncol.2008;30(1):29-34.[14]Morabito A,De ME, Di MM,et al.Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions.Oncologist.2006;11(7):753-764.[15]Zachary I.VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans.2003;31(6):1171-1177.[16]Vincenti V,Cassano C,Rocchi M,et al.Assignment of the vascular endothelial growth factor gene to human chromosome 6p21.3. Circulation.1996;93(8):1493-1495.[17]Koga K,Osuga YO,Hirota Y,et al.Elevated Serum Soluble Vascular Endothelial Growth Factor Receptor 1 (sVEGFR-1) Levels in Women with Preeclampsia.J Clin Endocrinol Metab.2003;88(5):2348.[18]Butkiewicz D, Krze?niak M, Drosik A,et al.The VEGFR2, COX-2 and MMP-2 polymorphisms are associated with clinical outcome of patients with inoperable non-small cell lung cancer.Int J Cancer. 2015;137(10): 2332-2342. [19]廖博,马保安,刘喆,等.骨肉瘤中血管内皮生长因子受体KDR的表达及其分析[J].第四军医大学学报,2013,24(20):1857-1859.[20]Hou WZ,Chen XL,Wu W,et al.MicroRNA-370-3p inhibits human vascular smooth muscle cell proliferation via targeting KDR/AKT signaling pathway in cerebral aneurysm. Eur Rev Med Pharmacol Sci. 2017;21(5):1080-1087.[21]Tai IT,Meiru D,Bo CL.Periostin induction in tumor cell line explants and inhibition of in vitro cell growth by anti-periostin antibodies. Carcinogenesis.2005;26(5):908.[22]Ouyang G,Liu M,Kai R,et al.Upregulated expression of periostin by hypoxia in non-small-cell lung cancer cells promotes cell survival via the Akt/PKB pathway.Cancer Lett.2009;281(2):213-219.[23]Oh HJ,Bae JM,Wen XY,et al. Overexpression of POSTN in Tumor Stroma Is a Poor Prognostic Indicator of Colorectal Cancer.J Pathol Transl Med.2017;51(3):306-313. [24]Shao R,Bao S,Bai X,et al.Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression.Mol Cell Biol. 2004;24(9):3992-4003.[25]Karar J,Maity A.PI3K/AKT/mTOR Pathway in Angiogenesis.Front Mol Neurosci.2011;4:51.[26]Kai R,Bao S,Ouyang G.The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci. 2009;66(14):2219-2230. |