Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (26): 4235-4240.doi: 10.3969/j.issn.2095-4344.0764
Previous Articles Next Articles
Physical guidance cues of nerve conduits
Xiang Yang1, Wu Yi-bing1, Wei Peng1, Yin Jun2, Dai Guang-li1
- 1Ningbo First Hospital, Ningbo 315000, Zhejiang Province, China; 2Zhejiang University, Hangzhou 310000, Zhejiang Province, China
-
Received:2017-12-23 -
Contact:Dai Guang-li, Engineer, Ningbo First Hospital, Ningbo 315000, Zhejiang Province, China -
About author:Xiang Yang, Master, Physician, Ningbo First Hospital, Ningbo 315000, Zhejiang Province, China -
Supported by:the National Natural Science Foundation of China, No. 11402056; the Second-Batch Scientific Research Program of Ningbo City, No. 2017A610215
CLC Number:
Cite this article
Xiang Yang, Wu Yi-bing, Wei Peng, Yin Jun, Dai Guang-li. Physical guidance cues of nerve conduits[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(26): 4235-4240.
share this article
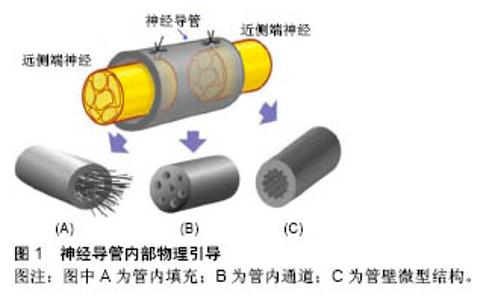
2.1 神经导管管壁物理性质 2.1.1 管壁孔隙 神经导管通透性对于神经再生至关重要,管壁应该具有半通透性,以便营养物质、氧气和生长因子扩散至管内,同时将废物排出至管外,有研究认为管壁应该能允许相对分子质量50 000的分子通 过[31]。管壁的通透性直接和管壁孔隙有关,孔隙具有促进细胞黏附、轴突再生、营养物质扩散等用[32]。孔隙大小发挥至关重要的影响,孔隙直径小于5 μm,则细胞和组织难以通过;孔隙直径大于30 μm,则会使得炎性细胞通过,进而阻碍神经再生。理想的孔隙直径应该在5-30 μm之间,更精确的说是在10-20 μm之间[30]。当前纳米制备技术的发展,使得管壁孔隙的大小能在微米甚至纳米级别上进行调控;而且促进了非对称管壁设计的完成,即管壁外层为微孔隙,内层为大孔隙,赋予导管选择性的双向通透性,阻止更多无用细胞进入,帮助废物排出。当许旺细胞和成纤维细胞分别播种于该导管管壁时,许旺细胞在管内获得增生,而成纤维细胞向管内生长则受到抑制[33]。 2.1.2 管壁机械性能 神经导管具有刚度、柔韧性、降解性等重要机械性能,这些性能决定导管能在机体内环境中保持完整,能在神经再生完成后被机体吸收,甚至能为神经再生提供合适的引导和支持。管壁刚度具有调整神经突生长张力的作用,基底刚度的增加也导致神经突上牵张力的增加,进而调整神经再生[34];在正常生理机制中,牵张力必须达到临界阈值,以激活神经再生[35]。有研究致力于探寻管壁最佳刚度,以便达到最适宜的牵张力,结论是存在最适合神经生长的管壁刚度[36],然而由于实验环境和测量终点的差异,最适宜刚度值未达到一致,根据管壁构成材料不同刚度值在5-50 Pa波动[37]。管壁物理顺应性对神经再生过程中细胞功能有重要作用,然而相关作用机制有待进一步研究[38-39]。管壁厚度通常被认为与营养物质扩散及管壁通透性有关,也可能对轴突再生产生影响,有研究证实管壁厚度超过 0.81 mm会减缓轴突生长[40];Kokai等[41]证实导管符合管壁厚度0.6 mm、孔隙率80%、孔隙直径10-40 μm,最适宜神经再生。 2.2 神经导管内部物理引导 如图1所示,神经导管内部物理引导方式可分为:管内填充、管内通道和管壁微型结构。"
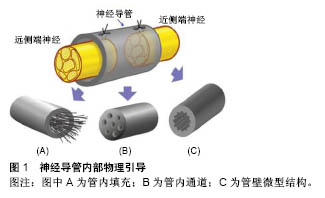
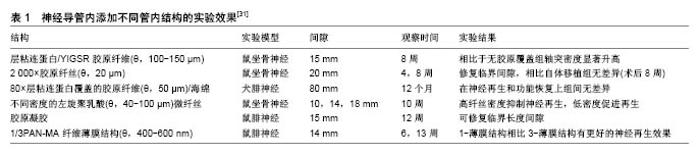
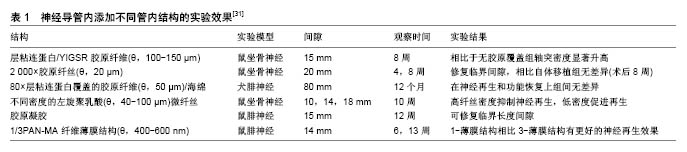
2.2.1 管内填充 神经导管腔内的结构设计需满足以下条件:易于支持细胞分散、促进损伤神经组织在三维空间内生长。一种方式是在导管内添加物理填充物,以使管内构造和自体神经相接近。填充物由生物材料构成,主要包括纤维、纤丝、薄膜、海绵和胶原(表1)。 纤维和纤丝束是最初引入管内的填充物,其在管内纵向并定向排列,对轴突再生起到定向引导作用;除了接触引导作用,纤维和纤丝同样增加了管腔的横断面积,增加有髓轴突生成,进而促进感觉功能恢复。多项研究证明相比于未添加纤维导管,管内添加纤维的导管具有更好的促神经再生作用[42-44];此外,纤维可以用于传递生长因子,从而在导管内创造浓度梯度,引导神经轴突沿浓度梯度迁移。有研究在以壳聚糖为材料的导管 内加入聚乙交酯纤丝,成功修复了狗坐骨神经30 mm的间隙[45],该导管内聚乙交酯纤丝起到促进许旺细胞迁移的作用,并且壳聚糖和聚乙交酯的降解产物分别为葡萄糖胺和酸性乙醇酸,酸碱中和后在体内代谢掉,维持内环境pH值的稳定。虽然纤维或纤丝具有促神经再生作用,但是管内过多的填充物反而阻碍再生轴突迁移,并且占据管内神经再生所需空间,所以纤维和纤丝作为填充物应保持适当浓度。 薄膜具有较高的表面积/体积比、紧凑的结构及较低的空间占用,通常每个薄膜只占了导管通道0.6%的空间,也称为薄膜的存储密度[46]。薄膜可在管道内呈现有序分布,避免了管道内引入纤维或纤丝时产生的交错折叠。薄膜具有明显的促进神经修复作用,但并非薄膜越多效果越好,研究证明相比于多薄膜,单薄膜具有更好的促神经修复效果,多薄膜会造成细胞混乱分布和排列,并且限制轴突生长,进而减慢神经传导速率[46]。 海绵是一种重要的填充物,有研究设计出一种以聚乙醇酸为材料的导管,内部填充有浸透层粘连蛋白的猪胶原海绵,管内径为3.0-4.0 mm,植入实验犬腓神经 80 mm间隙中,术后通过组织和电生理评估,证实该种导管甚至比自体神经移植有更好的促再生效果,尽管部分有神经瘤形成[47]。 胶原和层粘连蛋白通过形成供无神经元细胞迁移的基质,来促进神经修复。有研究证实相比于未填充的硅胶导管,在硅胶导管内填充胶原和包含层粘连蛋白的凝胶(包含层粘连蛋白)具有更好的神经修复效果[48],并且最多能修复15-20 mm的神经缺损[49]。当前,多种胶原和包含层粘连蛋白的凝胶被用于整合支持细胞和生长因子,并且层粘连蛋白-整合素活化位点衍生出的寡肽,其可能引导再生轴突的作用有待研究[50]。 2.2.2 管内通道 中空导管内设计通道是提高神经再生效果的一种方式,此种设计模仿神经中内环境,当管内包含定向的通道时,使得再生轴突沿着指定方向生长,同时限制轴突混乱扩散。最初认为该种设计通过增加许旺细胞黏附和分布的表面积,进而促进同种异体许旺细胞迁入[51],支持神经通过较大间隙再生,然而后续研究证明通道并非越多越好,相关研究采用单通道、2-通道、4-通道、7-通道导管在大鼠坐骨神经进行对比实验,结果显示多通道相比于单通道导管修复神经效果是相同的[52]。另外管内多通道侵占管道内部空间,可能会影响导管的部分特性,比如通透性、降解性、变型性、膨胀性等。所以多通道是否显著优于单通道导管还未有明确结论。 2.2.3 管壁微型结构 研究表明,神经细胞能够响应微米或者纳米尺寸的结构[53-54],在管壁表面添加该种物理因素,神经细胞不仅能识别该种结构特征,还能与表面形状发生作用,产生接触引导,促进神经再生。静电纺丝纳米纤维属于微型结构,通过静电纺丝技术使纳米结构和支架合成部分地模仿细胞外基质内环境,如此影响细胞迁移、轴突引导和再生。相比于单纯导管,静电纺丝纳米纤维增加了接触表面积,同时增加了表面拓扑性,进而促进神经再生,其优点总结如下[55]:材料弹性和渗透性好,在生物机体环境中较为适用;纳米和微米纤维具有较好的表面积/体积比,进而增加了管内物质作用面积,有利于蛋白质吸收、许旺细胞迁移和轴突再生;静电纺丝纤维定向排列,进而引导许旺细胞定向排列、移动和增殖,最终促进轴突再生[21,44]。Chew等[21,56]制造出一种聚己内酯-聚磷酸乙酯乙烯(PCLEEP)静电纺丝纤维导管,成功修复1.5 cm的神经间隙,相比于非静电纺丝纤维PCLEEP导管,具有更好的功能恢复效果,后者在添加外源性生长因子之后,神经再生效果显著提升。管壁微型结构的使用,避免和管内添加低密度引导结构一样,最终导致纤维分布不均匀,该种结构的优点是消除了由于纤维重叠或压缩造成的神经生长抑制[57],除此之外,静电纺丝导管本身可充当生长因子和其他再生分子的载体。 在神经导管内表面添加纵向排列的微型沟槽结构,是添加管壁微型物理结构的一种方法。这种方法被证明能更好地促进神经再生,Clements等[46]制造出一种胶原-壳聚糖混合材料导管,内壁添加定向的纵向微沟槽,在没有添加促神经因子、细胞等生物化学因素的前提下,成功修复了长度达到临界值1.5 cm的神经间隙,术后神经再生和功能恢复效果显著优于中空导管,并且和自体神经移植后12周的效果持平。沟槽深度影响轴突生长,Li和Folch等[58]证实胚胎鼠皮层神经元在22-69 μm深的沟槽边缘转向,但在2-5 μm的浅沟槽上下生长。沟槽的宽度同样影响神经突生长方向,沟槽宽度值通常在20-60 μm范围,Mahoney等[59]证实神经突生长方向倾向于和窄沟槽管壁平行,和较宽的沟槽(40-60 μm)管壁垂直。 一次成型制备内壁有纵向微沟槽的中空神经导管,可用浸没沉淀法。浸没沉淀法基于相转化原理,通过控制相转化的过程得到所需的平板膜或管状膜。浸没沉淀法在工业中应用非常普遍且成熟,而基于这种方法的纺丝技术—干/湿纺丝技术可用于制备中空管状膜,其过程为:将聚合物溶液和非溶剂(或其他溶液)分别用进样针注入纺丝器中,这两相不相容的溶液经过一段空气段或者其他控制气氛后浸入凝结池进行凝结,形成中空管状膜。有研究者将相对分子质量为730 000(ID=0.992 u)的消旋聚乳酸、溶剂、添加剂置入挤出器中溶解,除泡后用纺丝器制成外径为2.3 mm、内径为1.0 mm、壁厚为0.4 mm的管道,再经洗涤,去除添加剂,制成有孔的中空管[60]。干湿纺丝法是一种能够一次性成型制备内壁具有特定结构的聚合物神经导管的加工方法,并且能够通过对工艺参数的调整,有效改变和控制神经导管的渗透性、微观结构和机械强度等物理性质,制作便利且有可调控性。 "
| [1] Siemionow M,Brzezicki G.Chapter 8: Current techniques and concepts in peripheral nerve repair. Int Rev Neurobiol. 2009;87:141-172.[2] Neal S,Fields KB. Peripheral nerve entrapment and injury in the upper extremity.Am Fam Physician. 2010;81(2):147-155.[3] Noble J,Munro CA,Prasad VS,et al.Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries.J Trauma.1998;45:116-122.[4] Pabari A,Yang SY,Seifalian AM,et al.Modern surgical management of peripheral nerve gap.J Plast Reconstr Aesthet Surg. 2010;63: 1941-1948.[5] Tezcan AH.Peripheral Nerve Injury and Current Treatment Strategies. 2017.DOI: 10.5772/intechopen.68345[6] Guo D,Guo D,Guo J,et al.A Clinical Study of the Modified Thread Carpal Tunnel Release. Hand.2017;12:453.[7] Mietto BS,Mostacada K,Martinez AMB.Neurotrauma and inflammation: CNS and PNS responses.Mediators Inflamm. 2015;2015:1-14.[8] Seckel BR.Enhancement of peripheral nerve regeneration. Muscle Nerve.1990;13:785-800.[9] Gundersen RW,Barrett JN.Characterization of the turning response of dorsal root neurites toward nerve growth factor.J Cell Biol. 1980;87: 546-554.[10] Dodd J,Jessell TM.Axon guidance and the patterning of neuronal projections in vertebrates.Science.1988;242:692-699.[11] Chiono V,Tonda-Turo C.Trends in the design of nerve guidance channels in peripheral nerve tissue engineering. Prog Neurobiol. 2015;131:87-104.[12] Johnson EO,Soucacos PN.Nerve repair: experimental and clinical evaluation of biodegradable artificial nerve guides. Injury.2008;39 Suppl 3:S30-36.[13] Lin MY,Manzano G,Gupta R.Nerve allografts and conduits in peripheral nerve repair.Hand Clin. 2013;29:331-348.[14] Lad SP,Nathan JK,Schubert RD,et al.Trends in median, ulnar, radial, and brachioplexus nerve injuries in the United States. Neurosurgery. 2010;66:953-960.[15] Panseri S,Cunha C,Lowery J,et al.Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnol.2008;8:39.[16] Elkwood AI,Holland NR,Arbes SM,et al.Nerve allograft transplantation for functional restoration of the upper extremity: case series.J Spinal Cord Med.2011;34:241-247.[17] Muir D.The potentiation of peripheral nerve sheaths in regeneration and repair.Exp Neurol. 2010;223:102-111.[18] Mackinnon SE,Doolabh VB,Novak CB,et al.Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107: 1419-1429.[19] Karabekmez FE,Duymaz A,Moran SL.Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand.Hand(N Y).2009;4:245-249.[20] Kehoe S,Zhang X,Boyd D.FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy.Injury. 2012;43(5):553-572. [21] Chew SY,Mi R,Hoke A,et al.Aligned Protein-Polymer Composite Fibers Enhance Nerve Regeneration: A Potential Tissue-Engineering Platform.Adv Funct Mater. 2007;17:1288-1296.[22] Tan A,Rajadas J,Seifalian AM.Biochemical engineering nerve conduits using peptide amphiphiles. J Control Release. 2012;163:342-352.[23] Longo FM,Hayman EG,Davis GE,et al.Neurite-promoting factors and extracellular matrix components accumulating in vivo within nerve regeneration chambers.Brain Res.1984;309:105-117.[24] Schmidt CE,Leach JB.Neural tissue engineering: strategies for repair and regeneration.Annu Rev Biomed Eng. 2003;5:293-347.[25] Lundborg G,Hansson HA.Nerve regeneration through preformed pseudosynovial tubes A preliminary report of a new experimental model for studying the regeneration and reorganization capacity of peripheral nerve tissue.J Hand Surg.1980;5:35-38.[26] Luo L,Gan L,Liu Y,et al.Construction of nerve guide conduits from cellulose/soy protein composite membranes combined with Schwann cells and pyrroloquinoline quinone for the repair of peripheral nerve defect.Biochem Biophys Res Commun.2015;457(4):507-513. [27] Appel EA,del Barrio J,Loh XJ,et al.Supramolecular polymeric hydrogels.Chem Soc Rev. 2012;41:6195-214.[28] Sivolella S,Brunello G,Ferrarese N,et al.Nanostructured guidance for peripheral nerve injuries: a review with a perspective in the oral and maxillofacial area.Int J Mol Sci. 2014;15:3088-3117.[29] Deumens R,Bozkurt A,Meek MF,et al.Repairing injured peripheral nerves: Bridging the gap.Prog Neurobiol. 2010;92:245-276.[30] Jiang X,Lim SH,Mao HQ,et al.Current applications and future perspectives of artificial nerve conduits.Exp Neurol. 2010;223:86-101.[31] Daly W,Yao L,Zeugolis D,et al.A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery.J R Soc Interface. 2012;9:202-221.[32] O'Brien FJ,Harley BA,Waller MA,et al.The effect of pore size on permeability and cell attachment in collagen scaffolds for tissue engineering.Technol Health Care.2007;15:3-17.[33] Chang CJ,Hsu SH,Yen HJ,et al.Effects of unidirectional permeability in asymmetric poly( DL -lactic acid- co -glycolic acid) conduits on peripheral nerve regeneration: An in vitro and in vivo study. J Biomed Mater Res B Appl Biomater. 2007;83B:206-215.[34] Koch D,Rosoff WJ,Jiang J,et al.Strength in the Periphery: Growth Cone Biomechanics and Substrate Rigidity Response in Peripheral and Central Nervous System Neurons.Biophys J. 2012;102:452-460.[35] Leach JB,Brown XQ,Jacot JG,et al.Neurite outgrowth and branching of PC12 cells on very soft substrates sharply decreases below a threshold of substrate rigidity.J Neural Eng. 2007;4:26-34.[36] Gunn JW,Turner SD,Mann BK.Adhesive and mechanical properties of hydrogels influence neurite extension.J Biomed Mater Res A. 2005; 72(1):91-97.[37] Willits RK,Skornia SL.Effect of collagen gel stiffness on neurite extension.J Biomater Sci Polym Ed. 2004;15:1521-1531.[38] Engler AJ,Sen S,Sweeney HL,et al.Matrix elasticity directs stem cell lineage specification.Cell. 2006;126:677.[39] Mammoto A,Ingber DE.Cytoskeletal control of growth and cell fate switching.Curr Opin Cell Biol.2009;21:864-870.[40] Rutkowski GE,Heath CA.Development of a Bioartificial Nerve Graft. II. Nerve Regeneration in Vitro. Biotechnol Prog. 2002;18:373-379.[41] Kokai LE,Lin YC,Oyster NM,et al.Diffusion of soluble factors through degradable polymer nerve guides: Controlling manufacturing parameters. Acta Biomaterialia.2009;5:2540.[42] Wang HB,Mullins ME,Cregg JM,et al.Varying the diameter of aligned electrospun fibers alters neurite outgrowth and Schwann cell migration. Acta Biomater.2010;6:2970-2978.[43] Cho YI,Choi JS,Jeong SY,et al.Nerve growth factor (NGF)-conjugated electrospun nanostructures with topographical cues for neuronal differentiation of mesenchymal stem cells.Acta Biomater. 2010;6: 4725-4733.[44] Yang F,Murugan R,Wang S,et al.Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603-2610.[45] Wang X,Hu W,Cao Y,et al.Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft.Brain. 2005;128:1897-1910.[46] Clements IP,Kim YT,English AW,et al.Thin-film enhanced nerve guidance channels for peripheral nerve repair.Biomaterials. 2009;30: 3834-3846.[47] Inada Y,Morimoto S,Takakura Y,et al.Regeneration of peripheral nerve gaps with a polyglycolic acid-collagen tube.Neurosurgery. 2004;55: 640-646; discussion 6-8.[48] Madison RD,da Silva C,Dikkes P,et al.Peripheral nerve regeneration with entubulation repair: comparison of biodegradeable nerve guides versus polyethylene tubes and the effects of a laminin-containing gel.Exp Neurol. 1987;95:378-390.[49] Madison RD,Da Silva CF,Dikkes P.Entubulation repair with protein additives increases the maximum nerve gap distance successfully bridged with tubular prostheses.Brain Res. 1988;447:325-334.[50] Luo Y,Shoichet MS.A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat Mater. 2004;3: 249-253.[51] de Ruiter GC,Onyeneho IA,Liang ET,et al.Methods for in vitro characterization of multichannel nerve tubes.J Biomed Mater Res A. 2008;84:643-651.[52] Yao L,Ruiter GCW,Wang H,et al.Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit.Biomaterials. 2010;31:5789-5797.[53] Johansson F,Carlberg P,Danielsen N,et al.Axonal outgrowth on nano-imprinted patterns. Biomaterials.2006;27:1251-1258.[54] Cao H,Liu T,Chew SY.The application of nanofibrous scaffolds in neural tissue engineering. Adv Drug Deliv Rev. 2009;61:1055-1064.[55] Ramakrishna S,Joe R,Archana PS,et al.Science and engineering of electrospun nanofibers for advances in clean energy, water filtration, and regenerative medicine.J Mater Sci. 2010;45:6283-6312.[56] Chew SY,Wen J,Yim EK,et al.Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules. 2005;6: 2017-2024.[57] Stang F,Fansa H,Wolf G,et al.Structural parameters of collagen nerve grafts influence peripheral nerve regeneration. Biomaterials. 2005;26: 3083-3091.[58] Li N,Folch A.Integration of topographical and biochemical cues by axons during growth on microfabricated 3-D substrates.Exp Cell Res. 2005;311:307-316.[59] Mahoney MJ,Chen RR,Tan J,et al.The influence of microchannels on neurite growth and architecture. Biomaterials.2005;26:771.[60] 王光林,王翠莹.周围神经组织工程材料的预构[J].中国修复重建外科杂志, 2000,14(2):110-114. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||