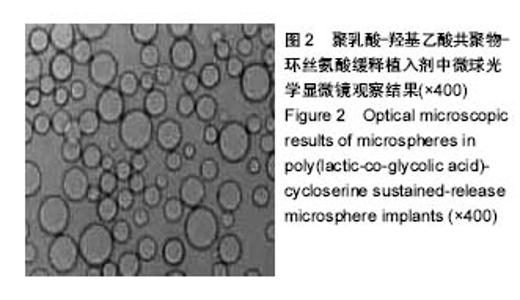
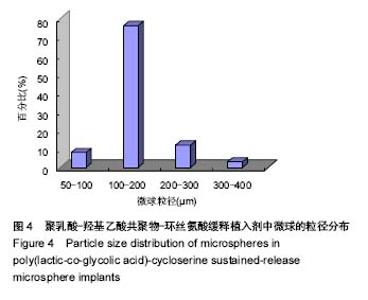
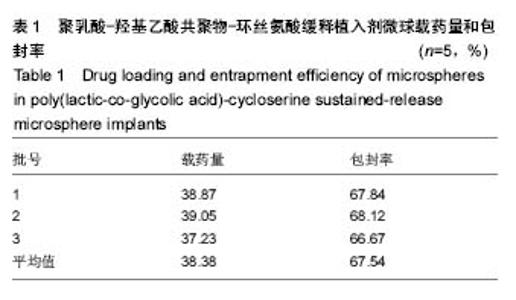
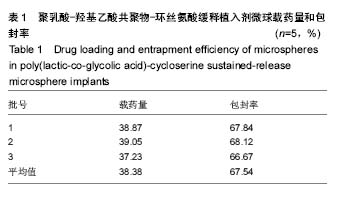
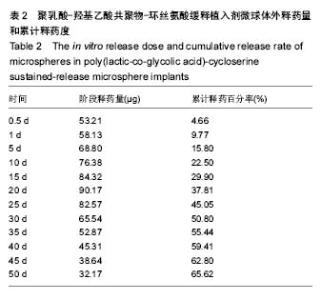
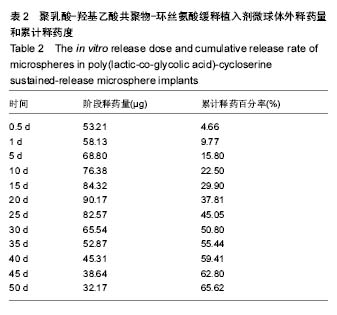
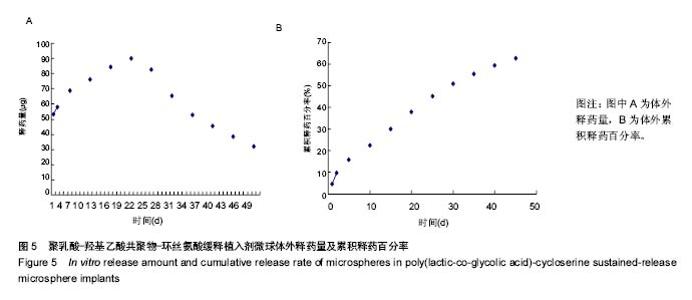
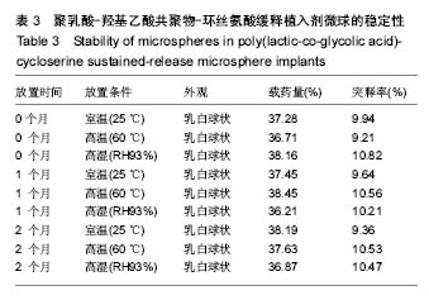
| [1]侯超.耐多药肺结核病人耐药情况及临床治疗转归分析[J].青岛大学医学院学报,2016,56(6):696-701.[2]李香社,祝玉芬.我国结核分枝杆菌耐药现状及研究进展[J].临床误诊误治,2017,30(7):114-117.[3]World Health Organizaition.Global Tuberculosis Report 2015[R].Geneva:World Health Organizaition,2015.[4]全国第五次结核病流行病学抽样调查技术指导组,全国第五次结核病流行病学抽样调查办公室.2010 年全国第五次结核病流行病学抽样调查报告[J].中国防痨杂志,2012,34(8):485-508.[5]张波,伊正君,付玉荣,等.分枝杆菌感染特征与结核分枝杆菌耐药性分析[J].中国病原生物学杂志,2015,10(8):685-688.[6]Zhang LX,Qin ZH,Sun HB.Multi-drug resistance of Mycobacterium tuberculosis strains in Tianjin,China from 2006 to 2015.Biomed Res.2017;28(10):4594-4598. [7]蒲新明,赛依旦•吐尔逊,阿尔泰,等.耐药结核病现状及研究进展[J].职业与健康,2016,32(1):717-720.[8]肖和平,方勇,范琳,等.耐药结核病化学治疗研究的过去、现状与未来[J].中国防痨杂志,2014,36(8):634-637.[9]黄建生,沈梅,孙亚玲,等.上海市肺外结核的流行病学分析[J].中华结核和呼吸杂志,2000,23(10):606-608. [10]王鑫,荆鑫,崔后春.脊柱结核的外科治疗及康复[J].中国矫形外科杂志,2005,13(24):1909-1910.[11]鲍玉成,张文龙.脊柱结核治疗进展[J].吉林医学, 2013,34(4): 714-716.[12]Bao YC,Li YL,Ning GZ,et al.Forked osteotomy arthroplasty for elbow tuberculosis: six years of follow-up.Eur J Orthop Surg Traumatol.2014;24(6):857-862.[13]Hong SJ,Yu HS,Kim HW.Tissue engineering polymeric mi-crocarriers with macroporous morphology and bone-bioactive surface.Macromol Biosci.2009;9(7):639-645.[14]裴洁,苏国生.联用环丝氨酸在耐多药肺结核治疗中的疗效及安全性比较[A].中华高血压杂志社.全国高血压防治知识推广培训班暨健康血压中国行海南海口会论文综合刊[C].中华高血压杂志社,2014:2.[15]沈莉莉.环丝氨酸治疗耐多药结核病的临床治疗效果[J].中国生化药物杂志,2017,37(8):193-197.[16]冯海燕,丛里龙,齐玉兰.环丝氨酸在治疗耐多药、广泛耐药肺结核中的应用[J].疾病监测与控制,2015,9(9):634-635.[17]辛朝雄,吴小霞,杨俭,等.含环丝氨酸联合胸腺肽方案治疗耐多药肺结核的效果[J].广东医学,2016,37(17):2646-2651.[18]赵冰,宋媛媛,逄宇,等.中国耐多药结核分枝杆菌二线抗结核药物敏感性分析[J].中国防痨杂志,2013,35(10):831-834.[19]阿孜古丽•依明.环丝氨酸治疗耐多药肺结核的不良反应分析[J].中国医药指南,2017,15(14):75-76.[20]冯海燕,丛里龙,齐玉兰.环丝氨酸在治疗耐多药、广泛耐药肺结核中的应用[J].疾病监测与控制,2015,9(9):634-635.[21]El-sayed L,Mohamed ZH,Wahbi AAM.Spectrophotometric and spectrofluorometric determination of cyclosrine with p- benzoquinone.Analyst.1986;111(8):915-917.[22]Wahbi AAM,Lotfi EA,Aboul EHY.Spectrophotometric determina-tion of tranexamic acid with chloranil.Talanta. 1984;31(1):77-78.[23]Atmaca S.Spectrophotometric determination of tranexamic acid with 2,4,6- trinitrobenzensulfonic acid.Acta pharm Turc. 1989;31(3):115-118.[24]Fattorini L.Activities of moxifloxacin alone and in combination with other antimicrobial agents against multi drug-resistant Mycobacterium tuberculosis infection in BALB/c mice. Antimicrob Agents Chemother.2003;47:360-362.[25]龙波,罗大勇,陈丹.含环丝氨酸治疗耐多药肺结核病患者的临床疗效[J].中国医药指南,2016,14(33):6-8.[26]Cycloserine.Tuberculosis(Edinb).2008;88:100-101.[27]余丽然,杨焕群,刘树周.环丝氨酸对结核分枝杆菌的耐药性及耐药机制探讨[J].海峡药学,2017,29(3):132-134.[28]王骞,刘海涛,施建党.聚乳酸/聚乙醇酸共聚物涂饰载三联抗结核药人工骨体外释药对比[J].中国组织工程研究, 2017,21(6): 911-916.[29]费正奇,胡藴玉.抗骨结核缓释药物:载体材料的选择[J].中国组织工程研究,2015,19(21):3387-3391.[30]缪昌东,姜继军.环丝氨酸致精神异常一例[J].中国防痨杂志, 2013,35(6):480-480.[31]Li K,Zhu M,Xu P,et al.Three-dimensionally plotted MBG/PHBHH composite scaffold for anti-tubercular drug delivery and tissue regeneration.Mater Sci Mater Med.2015; 26(2):1-8.[32]孙效虎,袁景,张宇.功能型骨组织工程支架修复结核性骨缺损[J].中国组织工程研究,2016, 20(30):4539-4545.[33]安森博,汪龙,胡懿郃.聚乳酸-羟基乙酸共聚物载药微球性质及缓释行为的影响因素[J].中国医院药学杂志, 2016,36(13): 1140-1144.[34]杨宗强,何胤,施建党,等.INH-PZA-PLGA 缓释微球制备及体外释药性能的研究[J].安徽医科大学学报,2016,51(1):9-13.[35]赵铭,郑启新,王金光.FCGH 仿生矿化对骨髓基质干细胞黏附率的影响[J].国际生物医学工程杂志,2006,29(1):1-4 |