Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (34): 5565-5570.doi: 10.3969/j.issn.2095-4344.2017.34.025
Previous Articles Next Articles
Articular cartilage defect repair with particulated juvenile cartilage allograft: existing problems and prospects
- 1Department of Joint Surgery, the Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; 2Department of Joint Surgery, Southwest Hospital, Third Military Medical University, Chongqing 400038, China
-
Received:2017-09-01Online:2017-12-08Published:2018-01-04 -
Contact:Liu Yi, Professor, Master’s supervisor, Department of Joint Surgery, the Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
About author:You Qi, Studying for master’s degree, Department of Joint Surgery, the Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
Supported by:the National Natural Science Foundation of China, No. 81071484; the Science & Technology Program of Guizhou Province, No. LH[2016]7477
CLC Number:
Cite this article
You Qi, Liu Yi, Duan Xiao-jun, Yang Liu, Li Yu-wan, Zhu Xi-zhong.
share this article
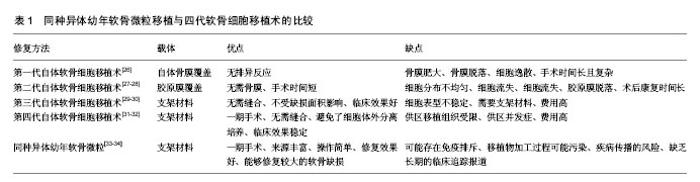
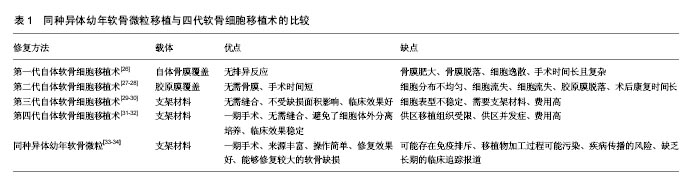
2.1 软骨缺损修复的常见方法 临床上治疗软骨缺损的方法很多,但每种方法均有一定的局限性,还不能很好的满足临床需要。①保守治疗虽能暂时缓解疼痛,但对于缺损直径大于4 mm的软骨损伤几乎无修复作用,更不能恢复其正常的生物学组织结构和功能;②关节镜下清理术只是对关节局部的清理,仅能改善症状并不能修复软骨缺损;③骨髓刺激术只适用于小的软骨缺损,形成的是纤维软骨,其结构组成和生物力学性能较透明软骨差[9]。而且微骨折术后常表现为软骨下骨过度增 生[10-11];④骨软骨移植术分为自体移植和同种异体移植,自体骨软骨移植为一期手术,手术操作简单,但软骨来源有限和供区并发症的缺点[12]。同种异体骨软骨移植可以修复较大的软骨缺损,但有免疫排斥和疾病传播的风险;⑤人工关节置换术面临术后骨溶解、远期关节功能退变以及费用高等问题;⑥应用新型生物材料修复关节软骨缺损,避免了软骨细胞提取、分离的复杂步骤,改善了关节的运动功能,但具有软骨修复不全、软骨形态改变、骨赘形成等缺点[13];⑦基因转染的软骨细胞修复关节软骨缺损,常用的转染因子为转化生长因子β和骨形态发生蛋白,该技术具有良好的软骨损伤修复效果,但基因长期稳定表达以及临床长期安全性等问题还需进一步解决[14]。以上治疗方法虽能暂时改善关节活动功能,但在长期观察中所出现的问题不容小觑。 2.2 同种异体幼年软骨微粒 不同于传统的异体骨软骨移植,同种异体幼年软骨微粒为纯软骨组织,不带有软骨下骨。首先获取异体幼年关节软骨(一般年龄<13岁),制成1 mm×1 mm×1 mm的软骨微粒备用,当手术确诊关节软骨缺损后,一期将软骨微粒复合纤维蛋白凝胶临时固定于软骨缺损处,然后进行康复训练。由美国Zimmer公司生产的DeNovo NT系统已经应用于临床,产品每个包装的DeNovo NT软骨来源于同一供体,一般包含30-200个颗粒,有效期为采集后45 d内[15]。幼年软骨微粒具有以下优点:①幼年软骨微粒中的软骨细胞增殖能力和体外形成组织工程软骨的能力强。Bonasia 等[16]将幼年软骨细胞、成年软骨细胞、1∶1比例混合的幼年软骨细胞和成年软骨细胞分别体外培养,6周后经生化检测和组织学检测,发现幼年软骨细胞的增殖明显好于成年软骨细胞和混合培养的细胞,而混合培养细胞和成年软骨细胞的增殖没有明显差别。Adkisson等[17]应用无血清培养基对软骨细胞进行一段时间的体外培养和生化检测,发现幼年软骨细胞产生的新生软骨蛋白聚糖含量是成熟软骨细胞的100倍,Ⅱ型胶原和Ⅸ型胶原mRNA表达量分别是成熟软骨细胞表达量的100倍和700倍,而且新生的Ⅱ型胶原和Ⅸ型胶原的分布和自体软骨组织胶原的分布相似。将幼年软骨细胞和成熟软骨细胞复合琼脂糖凝胶三维培养,发现幼年软骨细胞增殖速度比成熟软骨细胞增殖速度快。Smeriglio等[18]将幼年软骨细胞和成熟的软骨细胞复合仿生水凝胶在体外培养,3和6周后,发现幼年软骨细胞表达的成软骨基因比成熟软骨细胞显著升高,而且蛋白聚糖、Ⅱ型胶原表达量高于成熟软骨细胞。Skaalure等[19]将幼年软骨细胞和成熟软骨细胞复合水凝胶体外培养4周后,经免疫组化检测,发现幼年软骨细胞较成熟软骨细胞生成更多的胶原蛋白,而且成熟软骨细胞细胞外基质中有很多降解的胶原片段,通过Western blot检测发现成熟软骨细胞外基质中有很多降解的蛋白聚糖片段。由此得出幼年软骨具有较强的合成细胞外基质的能力,而且其降解代谢较成熟软骨低。据Marmotti等[20]报道,将幼年软骨微粒和成熟软骨微粒复合透明质酸支架体外培养,分别在1,2,3个月时进行细胞计数,发现幼年软骨微粒细胞增殖能力明显强于成熟软骨微粒,而且实验还证实转化生长因子β和粒细胞集落刺激因子能够促进幼年软骨微粒中细胞迁移和增殖;②幼年软骨的抗原性弱,体内移植不会引起强烈的排斥反应。Adkisson等[21]经体外实验发现幼年软骨细胞不会引起T细胞增殖,也不会表达引起T细胞免疫反应的细胞分子,如组织相容性复合体Ⅱ类抗原和共刺激分子B7-1和B7-2。此外,幼年软骨细胞能够表达抑制T细胞增殖的多种负性调节因子,如B7家族分子(B7-H1、B7-H2、B7-H3、B7-H4及B7-DC)、软骨调节素I和吲哚胺2,3-双加氧酶。Farr等[22]将同种异体软骨细胞和淋巴细胞进行体外共培养后,未发现淋巴细胞增殖现象,并且认为软骨细胞低表达除了能够引起免疫排斥反应的刺激分子如(CD80、CD86),还能够表达抑制淋巴细胞增殖的表面蛋白分子。Lu等[23]将牛和人的软骨微粒复合凝胶分别移植于SD大鼠的皮下,1个月后,移植的软骨微粒经过组织学染色和免疫组化鉴定结果显示软骨细胞能够存活并大量增殖而且产生了丰富的细胞外基质。在组织结构上,软骨组织缺乏血管、淋巴系统而且又具有致密、坚硬的细胞外基质,可以一定程度上避免宿主免疫细胞的攻击,所以这可能是其低免疫原性的原因之一[24];③同种异体幼年软骨微粒修复是比较理想的一期手术过程,不同于自体软骨细胞移植,DeNovo-NT有45 d的储存期,在关节镜下发现软骨缺损后即可行一期手术治疗[25]。 2.3 同种异体幼年软骨微粒移植与软骨细胞移植术的比较 软骨组织缺乏血管和神经,血液供应贫乏,因此,软骨组织损伤后很难自身修复。组织损伤的修复往往需要细胞的迁移和再生,由于坚硬的软骨细胞外基质的存在,周围软骨细胞很难迁移到软骨缺损区,因此,体内软骨细胞低下的迁移能力被认为是软骨缺损修复能力效果差的主要原因。1984年,Peterson应用兔自体软骨细胞移植治疗膝关节软骨缺损,开创了软骨细胞移植术。近年来,随着再生医学、材料学以及生物化学的发展,软骨细胞移植已经发展到第三代,即基质诱导的自体软骨细胞移植术。近期,有研究者采用患者自体非负重关节的软骨组织,切成大小为1.0-2.0 mm的软骨片,复合可生物降解的支架材料移植到软骨缺损处修复缺损,将该技术称为第四代自体软骨细胞移植技术。现将同种异体幼年软骨微粒移植和四代软骨细胞移植技术的优缺点归纳总结于表1。 2.4 同种异体幼年软骨微粒体的实验研究 1983年Albrecht等[35]开创了运用软骨微粒移植修复软骨缺损的技术,将获取的软骨微粒移植于兔直径为4 mm的软骨缺损处,术后40周,发现软骨微粒移植组有大量的软骨细胞增殖,并且有透明软骨生成。而空白对照组和仅使用纤维蛋白凝胶组损伤处未见透明软骨生成。又有学者用大鼠、山羊、马做了类似的实验,并取得了相似的实验结果[36]。同临床研究相比,近年来关于同种异体幼年软骨微粒基础研究的文献报道较少。Bonasia等[37]用成熟软骨微粒和幼年的软骨微粒以及混合的成熟的软骨微粒和幼年软骨微粒分别移植于成年的新西兰大白兔的股骨滑车缺损处,分别在术后3和6个月进行取材观察,通过免疫组化检查以及番红染色,发现幼年软骨微粒组和混合成年软骨微粒和幼年软骨微粒组蛋白聚糖和Ⅱ型胶原的生成的量比成年软骨微粒组明显增多,而且新生的透明软骨样组织也较成年软骨微粒多,而幼年软骨微粒组和混合培养的软骨微粒组之间没有明显区别。 2.5 同种异体软骨微粒临床应用 同种异体幼年软骨微粒移植修复关节软骨缺损,在美国临床已经取得重要进展,其潜在的优越性能已逐渐被医生及患者接受。而国内目前尚无生产此类产品的技术,Denovo-NT还未进入中国市场。 目前关于幼年软骨微粒移植修复关节软骨缺损的文献报道还不是很多,其长期有效性尚缺乏大量的、系统的临床数据支持,需要进一步发展与完善。Farr等[38]率先报道采用同种异体幼年软骨微粒复合纤维蛋白凝胶修复膝关节软骨缺损,术后24个月,核磁共振显示患者关节软骨缺损得到修复,临床症状获得改善。后来又进行了前瞻性对照研究,结果表明术后患者膝关节疼痛减轻、运动功能明显改善,部分患者经历了2次膝关节镜探查并取材分析,初步疗效满意。同膝关节其他部位缺损的治疗效果相比,传统的治疗方法对于髌骨缺损的治疗效果欠佳,而同种异体幼年软骨微粒对于髌骨缺损的治疗却具有非常好的效果[39]。Arshi等[40],Buckwalter等[41]的近期研究也得出相似的结论。Mcmillan等[42]报道了在全关节镜下应用幼年软骨微粒修复全层股骨滑车缺损,避免了传统膝关节手术所引起的并发症,但该技术缺少专门的工具能够使移植物在缺损处分布均匀,而且移植材料或纤维蛋白凝胶有溶解可能。Hatic等[43]、Kruse等[44]相继报道了应用DeNovo-RT治疗踝关节软骨缺损的患者,术后随访证实,踝关节疼痛症状消失,软骨缺损修复良好,踝关节运动功能得到改善。其中Saltzman等[34]对33例用同种异体幼年软骨微粒治疗的踝关节软骨缺损的患者进行了回顾性研究,结果显示所有的患者疼痛减轻,踝关节活动改善,踝关节功能提高。但有6例患者需要在术后15个月进行非翻修型手术,有3例患者在术后3个月至2年间,有持续性软骨水肿和软骨表面不光滑现象,但所有患者在术中和术后并没有出现其他并发症。由于病例总数少而且随访时间短,目前尚无详细的远期随访报道,因此对于其远期疗效还需进一步观察。同种异体软骨微粒移植修复关节软骨缺损在其他关节也有报道,如肘关节、髋关节。Dunn等[45]报道用同种异体幼年软骨微粒移植修复肘关节软骨缺损,术后6个月随访,肘关节疼痛减轻,功能改善,MRI检查显示软骨缺损得到修复。Pascual-Garrido等[46]报道在关节镜下应用同种异体幼年软骨微粒修复髋关节软骨缺损,术后12个月随访证实,髋关节活动功能明显改善,疼痛消失,软骨缺损得到修复。同种异体幼年软骨微粒移植对于中度的髋关节软骨缺损(2-5 cm2)有较好的修复效果,但缺乏长期的临床追踪报道结果,因此,还不能对其远期疗效做出客观评价。 2.6 同种异体幼年软骨微粒移植存在的问题 同种异体幼年软骨微粒已经作为产品应用于临床,并已经获得FDA批准,但也存在移植物加工过程污染、疾病传播的风 险[47]。虽然在传统观点中可以用射线对物品进行消毒灭菌,但这不能用于软骨组织,因为此过程会很大程度地破坏软骨组织的生物学活性[48]。目前还没有找到好的方法用于软骨组织提取、移植过程的消毒灭菌。同时该技术的修复效果与患者的年龄也有一定关系,一般患者年龄在小于55岁有较好的效果,这可能是因为随着年龄的变化,关节腔内的生理环境也会发生相应变化,从而会影响软骨缺损的修复。同种异体幼年软骨微粒移植修复软骨缺损的效果是多因素影响的过程,例如软骨微粒的大小、复合支架材料的接种密度、支架材料的结构和生化性能都可能影响修复的结果,因此未来还需结合材料、体内修复机制等对其进一步研究。 "
| [1]Kontturi LS, Jarvinen E, Muhonen V, et al. An injectable, in situ forming type II collagen/hyaluronic acid hydrogel vehicle for chondrocyte delivery in cartilage tissue engineering. Drug Deliv Transl Res. 2014;4(2):149-158.[2]Temenoff JS, Mikos AG. Review: tissue engineering for regeneration of articular cartilage. Biomaterials. 2000;21(5): 431-440.[3]Schindler OS. Current concepts of articular cartilage repair. Acta Orthop Belg. 2011;77(6):709-726.[4]Montgomery SR, Foster BD, Ngo SS, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2070-2075.[5]Mehrotra C, Remington PL, Naimi TS, et al. Trends in total knee replacement surgeries and implications for public health, 1990-2000. Public Health Rep. 2005;120(3):278-282.[6]Makris EA, Gomoll AH, Malizos KN, et al. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11(1):21-34.[7]Riboh JC, Cole BJ, Farr J. Particulated articular cartilage for symptomatic chondral defects of the knee. Curr Rev Musculoskelet Med. 2015;8(4):429-435.[8]Bonasia DE, Marmotti A, Rosso F, et al. Use of chondral fragments for one stage cartilage repair: a systematic review. World J Orthop. 2015;6(11):1006.[9]Min BH, Choi WH, Lee YS, et al. Effect of different bone marrow stimulation techniques (BSTs) on MSCs mobilization. J Orthop Res. 2013;31(11):1814-1819.[10]Brown WE, Potter HG, Marx RG, et al. Magnetic resonance imaging appearance of cartilage repair in the knee. Clin Orthop Relat Res.2004;422:214-223.[11]Minas T, Gomoll AH, Rosenberger R, et al. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902-908.[12]Bedi A, Feeley BT, Williams RJ. Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010; 92(4):994-1009.[13]Filardo G, Kon E, Di MA, et al. Treatment of knee osteochondritis dissecans with a cell-free biomimetic osteochondral scaffold: clinical and imaging evaluation at 2-year follow-up. Am J Sports Med. 2013;41(8):1786-1793.[14]Che JH, Zhang ZR, Li GZ, et al. Application of tissue-engineered cartilage with BMP-7 gene to repair knee joint cartilage injury in rabbits. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):496-503.[15]陈旭旭,李箭,王涛,等.颗粒软骨移植技术研究进展[J].中国运动医学杂志,2016,35(07):687-689.[16]Bonasia DE, Martin JA, Marmotti A, et al. Cocultures of adult and juvenile chondrocytes compared with adult and juvenile chondral fragments: in vitro matrix production. Am J Sports Med. 2011;39(11):2355-2361.[17]Adkisson HD, Martin JA, Amendola RL, et al. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am J Sports Med. 2010;38(7):1324-1333.[18]Smeriglio P, Lai JH, Dhulipala L, et al. Comparative potential of juvenile and adult human articular chondrocytes for cartilage tissue formation in three-dimensional biomimetic hydrogels. Tissue Eng Part A. 2015;21(1-2):147-155.[19]Skaalure SC, Milligan IL, Bryant SJ. Age impacts extracellular matrix metabolism in chondrocytes encapsulated in degradable hydrogels. Biol Mate. 2012;7(2):024111.[20]Marmotti A, Bonasia DE, Bruzzone M, et al. Human cartilage fragments in a composite scaffold for single-stage cartilage repair: an in vitro study of the chondrocyte migration and the influence of TGF-beta1 and G-CSF. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1819-1833.[21]Adkisson HD, Milliman C, Zhang X, et al. Immune evasion by neocartilage-derived chondrocytes: Implications for biologic repair of joint articular cartilage. Stem Cell Res. 2010;4(1):57-68.[22]Farr J, Yao JQ. Chondral Defect Repair with Particulated Juvenile Cartilage Allograft. Cartilage. 2011;2(4):346-353.[23]Lu Y, Dhanaraj S, Wang Z, et al. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24(6):1261-1270.[24]Tompkins M, Adkisson HD, Bonner KF. DeNovo NT Allograft. Oper Tech Sports Med. 2013;21(2):82-89.[25]Yanke AB, Chubinskaya S. The state of cartilage regeneration: current and future technologies. Curr Rev Musculoskelet Med. 2015;8(1):1-8.[26]Peterson L, Minas T, Brittberg M, et al. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212-214.[27]Haddo O, Mahroof S, Higgs D, et al. The use of chondrogide membrane in autologous chondrocyte implantation. The Knee. 2004;11(1):51-55.[28]Filardo G, Kon E, Di Martino A, et al. Second-generation arthroscopic autologous chondrocyte implantation for the treatment of degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1704-1713.[29]Kon E, Delcogliano M, Filardo G, et al. A novel nano-composite multi-layered biomaterial for treatment of osteochondral lesions: technique note and an early stability pilot clinical trial. Injury. 2010;41(7):693-701.[30]Gille J, Behrens P, Schulz AP, et al. Matrix-Associated Autologous Chondrocyte Implantation: A Clinical Follow-Up at 15 Years. Cartilage. 2016;7(4):309-315.[31]Cole BJ, Farr J, Winalski CS, et al. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1170-1179.[32]Spalding T, Almqvist F, Brittberg M, et al. Biodegradable polyurethane meniscal scaffold for isolated partial lesions or as combined procedure for knees with multiple comorbidities: clinical results at 2 years. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):128-134.[33]Suarez LS, Richmond JC. Overview of procurement, processing, and sterilization of soft tissue allografts for sports medicine. Sports Med Arthrosc. 2007;15(3):106-113.[34]Saltzman BM, Lin J, Lee S. Particulated juvenile articular cartilage allograft transplantation for osteochondral talar lesions. Cartilage. 2017;8(1):61.[35]Albrecht F, Roessner A, Zimmermann E. Closure of osteochondral lesions using chondral fragments and fibrin adhesive. Arch Orthop Trauma Surg. 1983;101(3):213.[36]Frisbie DD, Lu Y, Kawcak CE, et al. In vivo evaluation of autologous cartilage fragment-loaded scaffolds implanted into equine articular defects and compared with autologous chondrocyte implantation. Am J Sports Med. 2009;37(Suppl 1): 71-80.[37]Bonasia DE, Martin JA, Marmotti A, et al. The use of autologous adult, allogenic juvenile, and combined juvenile-adult cartilage fragments for the repair of chondral defects. Knee Surg Sports Traumatol Arthrosc. 2016;24(12): 3988-3996.[38]Farr J, Tabet SK, Margerrison E, et al. Clinical, Radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med. 2014;42(6):1417.[39]Tompkins M, Hamann JC, Diduch DR, et al. Preliminary results of a novel single-stage cartilage restoration technique: particulated juvenile articular cartilage allograft for chondral defects of the patella. Arthroscopy. 2013;29(10):1661-1670.[40]Arshi A, Wang D, Jones KJ. Combined particulated juvenile cartilage allograft transplantation and medial patellofemoral ligament reconstruction for symptomatic chondral defects in the setting of recurrent patellar instability. Arthrosc Tech. 2016;5(5):e1149-1154.[41]Buckwalter JA, Bowman GN, Albright JP, et al. Clinical outcomes of patellar chondral lesions treated with juvenile particulated cartilage allografts. Iowa Orthop J. 2013;34: 44-49.[42]Mcmillan S, Lichtman GT, Betz C. All-arthroscopic implantation of minced juvenile chondral allograft for an isolated, full-thickness chondral lesion in the trochlea of an adult knee. Arthrosc Tech. 2016;5(2):e397-401.[43]Hatic SO, Berlet GC. Particulated juvenile articular cartilage graft (DeNovo NT Graft) for treatment of osteochondral lesions of the talus. Foot Ankle Spec. 2010;3(6):361-364.[44]Kruse DL, Ng A, Paden M, et al. Arthroscopic De Novo NT (R) juvenile allograft cartilage implantation in the talus: a case presentation. J Foot Ankle Surg. 2012;51(2):218.[45]Dunn JC, Kusnezov N, Orr J, et al. Osteochondral defects of the upper extremity treated with particulated juvenile cartilage transfer. HAND. 2015;10(4):683-687.[46]Pascual-Garrido C, Hao J, Schrock J, et al. Arthroscopic juvenile allograft cartilage implantation for cartilage lesions of the hip. Arthrosc Tech. 2016;5(4):e929-933.[47]Mcallister DR, Joyce MJ, Mann BJ, et al. Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2007;35(12):2148-2158.[48]Dziedzic-Goclawska A, Kaminski A, Uhrynowska-Tyszkiewicz I, et al. Irradiation as a safety procedure in tissue banking. Cell and tissue banking. 2005;6(3):201-219. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [4] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [5] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [6] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [9] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [10] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [11] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [12] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [13] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| [14] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| [15] | Fang Xiaoyang, Tang Tian, Wang Nan, Qian Yuzhang, Xie Lin. Repair and regenerative therapies of the annulus fibrosus [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1582-1587. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||