Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (33): 5360-5368.doi: 10.3969/j.issn.2095-4344.2017.33.018
Previous Articles Next Articles
Functional differentiation of dopaminergic neurons derived from human embryonic stem cells
Peng Ya-nan1, Hu Lan2, Wang Tan1, Li Ke3, Yang Liu1, Chen Li1, Chen Xiao-wu1, Chen Zhi-bin1,Zhao Zhen-qiang1
- 1Department of Neurology, 2Clinical Laboratory, 3Department of Ophtalmology, First Affiliated Hospital, Hainan Medical University, Haikou 570102, Hainan Province, China
-
Revised:2017-09-12Online:2017-11-28Published:2017-12-01 -
Contact:Zhao Zhen-qiang, M.D., Chief physician, Department of Neurology, First Affiliated Hospital, Hainan Medical University, Haikou 570102, Hainan Province, China -
About author:Peng Ya-nan, Master, Physician, Department of Neurology, First Affiliated Hospital, Hainan Medical University, Haikou 570102, Hainan Province, China -
Supported by:the National Natural Science Foundation of China, No. 3126023/C100308; the International Cooperation Projects of Hainan Province, No. KJHZ2015-09, 2012-GH002; Hainan Province Graduate Student Innovation Research Project, No. Hys2016-84
CLC Number:
Cite this article
Peng Ya-nan, Hu Lan, Wang Tan, Li Ke, Yang Liu, Chen Li, Chen Xiao-wu, Chen Zhi-bin,Zhao Zhen-qiang. Functional differentiation of dopaminergic neurons derived from human embryonic stem cells[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(33): 5360-5368.
share this article
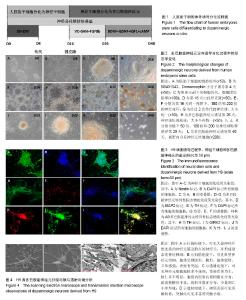
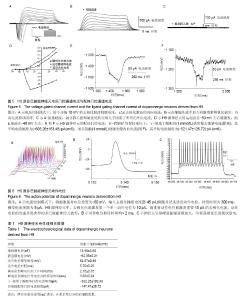
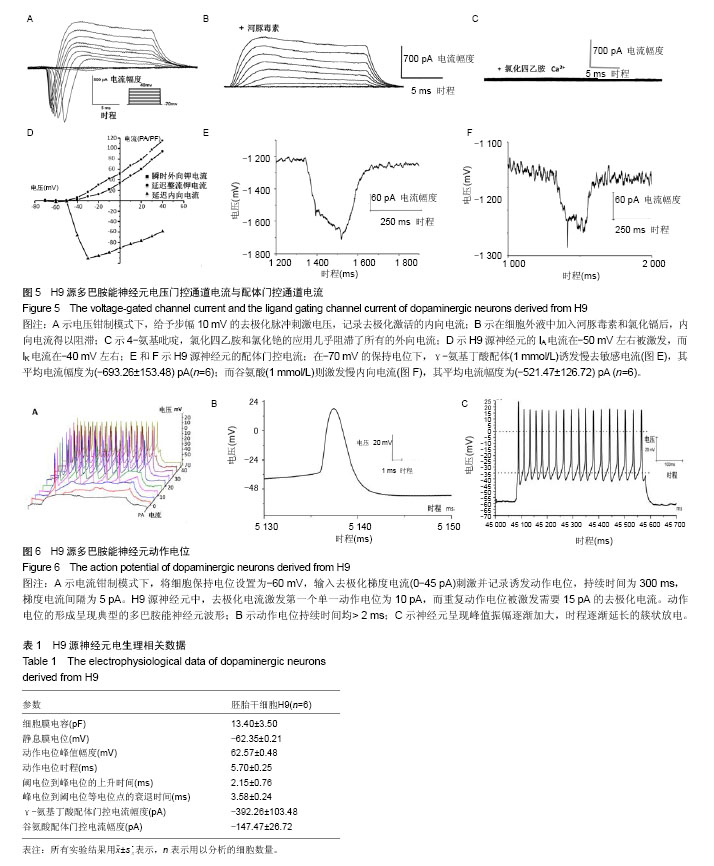
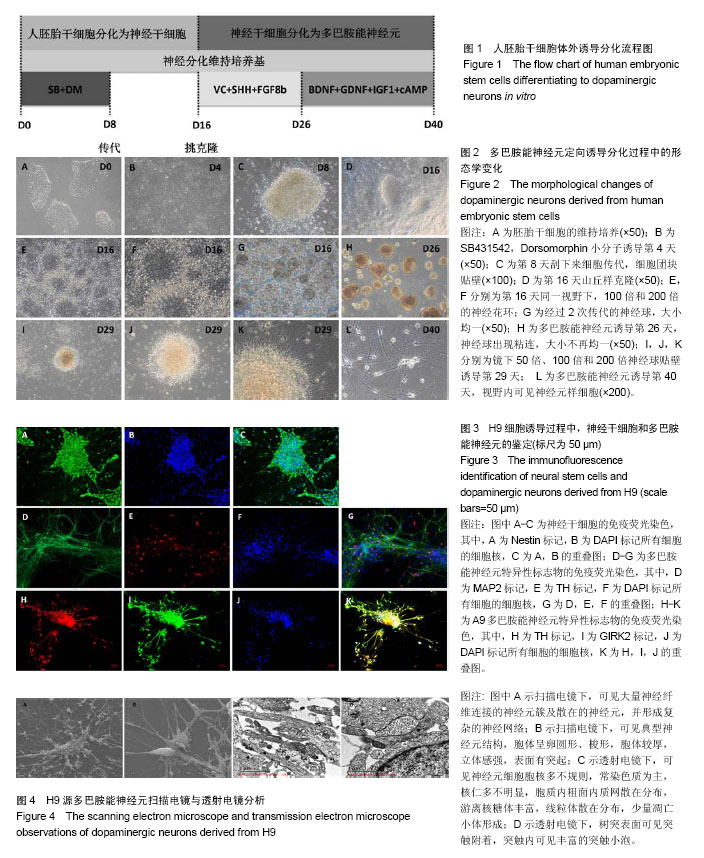
2.1 人胚胎干细胞体外诱导方案以及分化各阶段细胞形态变化 采用SMAD通道双抑制剂分化方案,体外诱导流程如图1。诱导前镜下观察胚胎干细胞大小形状均一,胞质透亮,呈铺路石样,伴高核质比(图2A)。用SB431542(SB)和Dorsomorphin(DM)诱导后,细胞逐渐变小变圆且更为紧密(图2B);传代后,细胞团块样贴壁(图2C);第16天可观察到神经花环(图2E,2F)或者山丘样克隆(图2D)。人工挑克隆悬球,经过2次传代,神经球进一步纯化(图2G);将神经球置于神经元诱导分化培养基培养10 d,此时神经球容易粘连,大小不再均一(图2H);分化终末期,神经球进一步贴壁分化(图2I,2J,2K),14 d后镜下可见神经样细胞(图2L)。 2.2 诱导分化过程中细胞免疫荧光鉴定 实验主要诱导过程分为2个阶段:胚胎干细胞分化为神经干细胞,神经干细胞分化为神经元。第一阶段选择了Nestin标记神经干细胞,第二阶段选择TH,GIRK2,MAP2标记多巴胺能神经元(图3)。第一阶段终末的神经球贴于玻片上,在细胞免疫荧光实验中,表达神经干细胞标志物Nestin(图3A,第16天)。第二阶段分化终末,细胞聚集成簇,从不同神经元簇发出的神经突形成复杂的神经网络(图3D-K)。实验观察到分化终末细胞在免疫荧光染色中表达神经元标志物MAP2(图3D),同时,在这些MAP2标记阳性的细胞中,存在一定数量的多巴胺能标志物TH的阳性标记(图3E),因此作者认为,这些MAP2/TH双阳性细胞(图3G)为多巴胺能神经元;同样,发现表达多巴胺能标志物TH的神经元(图3H),同时存在一定数量的GIRK2阳性标记(图3I),由于GIRK2为A9多巴胺能神经元的标志物,这些TH/GIRK2双阳性细胞(图3K)为A9多巴胺能神经元。实验选取10个随机镜下视野,分别对第一阶段的Nestin阳性细胞,第二阶段的MAP2/TH和TH/GIRK2双阳性细胞进行计数,对比DAPI标记的细胞总数,得出H9来源的神经干细胞、多巴胺能神经元和A9多巴胺能神经元的分化效率分别为52%,28%和18%。 2.3 多巴胺能神经元的形态学和超微结构 通过扫描电镜和透射电镜检测,展现分化产物神经元的形态学和超微结构。扫描电镜显示,H9源神经元均可见大量神经纤维连接的神经元簇及散在的神经元,形成复杂的神经网络(图4A)。在多巴胺能神经元分化终末,可以看到典型的神经元结构,细胞体呈卵圆形、梭形,胞体较厚,立体感强,表面有突起(图4B)。透射电镜检测主要观察分化终末的多巴胺能神经元内部的超微结构及相互的突触连接。镜下可见神经元细胞胞核多不规则,常染色质为主,核仁多不明显,胞质内粗面内质网散在分布,部分细胞可见粗面内质网丰富、轻度扩张。游离核糖体丰富,线粒体散在分布,部分线粒体肿胀、嵴断裂、减少。部分细胞核染色质凝集成块,可见凋亡小体形成(图4C)。H9来源神经元的典型结构主要包括:细胞器、细胞核、核糖体、神经丝和神经突触,在本实验中,观察到典型的突触结构和大量的突触囊泡(图4D)。这些结果证实了本实验分化所得的神经元与多巴胺能神经元的形态学和超微结构相符。 2.4 膜片钳检测 进一步检测了胚胎干细胞来源的多巴胺能神经元在体外的功能特性。采用膜片钳技术,检测神经元的全细胞电压门控电流、配体门控电流和动作电位。 2.4.1 电压门控电流 H9源神经元的细胞膜电容(Cm)平均为(13.40±3.5) pF(n=6),见表1。同时,记录该神经元的电压门控电流。电压钳制模式下,给予去极化脉冲刺激电压(脉冲幅度为-70 mV至+40 mV,步幅+10 mV,脉冲宽度50 ms),记录去极化激活的内向电流(图5A)。记录后,在细胞外液中加入河豚毒素(TTX)和氯化镉(CdCl2)用以阻滞内向电流(图5B),而4-氨基吡啶(4-AP),氯化四乙胺(TEA-Cl)和氯化铯(CsCl)的应用几乎阻滞了所有的外向电流(图5C)。这些结果证明神经元存在电压门控的钠电流(INa currents),瞬时外向钾电流(IA currents)和内向整流钾电流(IK currents)。基于细胞膜电容,依据细胞大小对电流幅度标准化(pA/pF,电流密度)。在H9源多巴胺能神经元中,INa电流平均在-50 mV或-60 mV左右出现,-30 mV或-20 mV左右达到峰值(图5D)。H9源神经元的峰值电流密度平均为(-112.97±22.54) pA/pF(n=6)。在所有的H9源神经元中,IA电流在-50 mV左右被激发,而IK电流在-40 mV左右(图5D)。IA和IK在40 mV的平均电流密度分别为(103.08±22.27) pA/pF(n=6),(81.37±18.68) pA/pF (n=6)。 2.4.2 配体门控电流 在-70 mV的保持电位下,γ-氨基丁酸配体(1 mmol/L)诱发慢去敏感电流,其平均电流幅度为(-392.26±103.48) pA (n=6,图5E);而谷氨酸(1 mmol/L)则激发慢内向电流(图5F),其平均电流幅度为(-147.47± 26.72) pA (n=6)。以上数据都表明H9源神经元在体外存在成熟有功能的γ-氨基丁酸和谷氨酸受体。 2.4.3 动作电位(APs) 电流钳制模式下,将细胞保持电位设置为-60 mV,输入去极化梯度电流(0-45 pA)刺激并记录诱发动作电位,持续时间为300 ms,梯度电流间隔为5 pA。对于H9源神经元而言,去极化电流激发第一个单一动作电位为10 pA,而重复动作电位被激发需要15 pA的去极化电流(图6A)。动作电位的形成呈现典型的多巴胺能神经元波形,动作电位持续时间均> 2 ms(图6B)。同时,神经元呈现峰值振幅逐渐加大,时程逐渐延长的簇状放电(图6C)。"
| [1] Spillantini MG, Schmidt ML, Lee VM, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839-840. ?[2] Damier P, Hirsch EC, Agid Y, et al. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122 ( Pt 8): 1437-1448. ?[3] Zhang Q, Chen W, Tan S, et al. Stem Cells for Modeling and Therapy of Parkinson's Disease. Hum Gene Ther. 2017;28(1): 85-98.[4] Jenner P. Dopamine agonists, receptor selectivity and dyskinesia induction in Parkinson's disease. Curr Opin Neurol. 2003;16 Suppl 1:S3-7.[5] Huot P, Johnston TH, Koprich JB, et al. The pharmacology of L-DOPA-induced dyskinesia in Parkinson's disease. Pharmacol Rev. 2013;65(1):171-222.[6] Barker RA, Drouin-Ouellet J, Parmar M. Cell-based therapies for Parkinson disease—past insights and future potential. Nat Rev Neurol. 2015;11(9):492-503.[7] Ziavra D, Makri G, Giompres P, et al. Neural stem cells transplanted in a mouse model of Parkinson's disease differentiate to neuronal phenotypes and reduce rotational deficit. CNS Neurol Disord Drug Targets. 2012;11(7):829-835.[8] Muraoka K, Shingo T, Yasuhara T, et al. Comparison of the therapeutic potential of adult and embryonic neural precursor cells in a rat model of Parkinson disease. J Neurosurg. 2008; 108(1):149-159.[9] He XB, Kim M, Kim SY, et al. Vitamin C facilitates dopamine neuron differentiation in fetal midbrain through TET1- and JMJD3-dependent epigenetic control manner. Stem Cells. 2015;33(4):1320-1332.[10] Kim JH, Auerbach JM, Rodríguez-Gómez JA, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418(6893):50-56.[11] Dahlstroem A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. i. demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand Suppl. 1964:SUPPL 232:1-55.[12] Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373(9680):2055-2066. ?[13] Tzschentke TM, Schmidt WJ. Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area in locomotion and reward. Crit Rev Neurobiol. 2000;14(2):131-142.[14] Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4(11): 2877-2890.[15] Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984;4(11): 2866-2876.[16] Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9(10):3463-3481.[17] Hainsworth AH, Röper J, Kapoor R, et al. Identification and electrophysiology of isolated pars compacta neurons from guinea-pig substantia nigra. Neuroscience. 1991;43(1):81-93.[18] Ungless MA, Grace AA. Are you or aren't you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35(7):422-430.[19] Good CH, Hoffman AF, Hoffer BJ, et al. Impaired nigrostriatal function precedes behavioral deficits in a genetic mitochondrial model of Parkinson's disease. FASEB J. 2011;25(4):1333-1344.[20] Branch SY, Chen C, Sharma R, et al. Dopaminergic Neurons Exhibit an Age-Dependent Decline in Electrophysiological Parameters in the MitoPark Mouse Model of Parkinson's Disease. J Neurosci. 2016;36(14):4026-4037.[21] Oh JE, Bae GU, Yang YJ, et al. Cdo promotes neuronal differentiation via activation of the p38 mitogen-activated protein kinase pathway. FASEB J. 2009;23(7):2088-2099.[22] Lee HS, Bae EJ, Yi SH, et al. Foxa2 and Nurr1 synergistically yield A9 nigral dopamine neurons exhibiting improved differentiation, function, and cell survival. Stem Cells. 2010;28(3): 501-512.[23] Takaesu G, Kang JS, Bae GU, et al. Activation of p38alpha/beta MAPK in myogenesis via binding of the scaffold protein JLP to the cell surface protein Cdo. J Cell Biol. 2006;175(3):383-388.[24] Perrier AL, Tabar V, Barberi T, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101(34):12543-12548.[25] Park CH, Minn YK, Lee JY, et al. In vitro and in vivo analyses of human embryonic stem cell-derived dopamine neurons. J Neurochem. 2005;92(5):1265-1276.[26] Buytaert-Hoefen KA, Alvarez E, Freed CR. Generation of tyrosine hydroxylase positive neurons from human embryonic stem cells after coculture with cellular substrates and exposure to GDNF. Stem Cells. 2004;22(5):669-674.[27] Zeng X, Cai J, Chen J, et al. Dopaminergic differentiation of human embryonic stem cells. Stem Cells. 2004;22(6):925-940.[28] Yan Y, Yang D, Zarnowska ED, et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23(6):781-790.[29] Schulz TC, Noggle SA, Palmarini GM, et al. Differentiation of human embryonic stem cells to dopaminergic neurons in serum-free suspension culture. Stem Cells. 2004;22(7): 1218-1238.[30] Park S, Lee KS, Lee YJ, et al. Generation of dopaminergic neurons in vitro from human embryonic stem cells treated with neurotrophic factors. Neurosci Lett. 2004;359(1-2):99-103.[31] Cho MS, Lee YE, Kim JY, et al. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105(9): 3392-3397.[32] Yang F, Liu Y, Tu J, et al. Activated astrocytes enhance the dopaminergic differentiation of stem cells and promote brain repair through bFGF. Nat Commun. 2014;5:5627.[33] Bayly RD, Brown CY, Agarwala S. A novel role for FOXA2 and SHH in organizing midbrain signaling centers. Dev Biol. 2012;369(1):32-42.[34] Andersson E, Tryggvason U, Deng Q, et al. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124(2):393-405.[35] L'Episcopo F, Tirolo C, Testa N, et al. Wnt/β-catenin signaling is required to rescue midbrain dopaminergic progenitors and promote neurorepair in ageing mouse model of Parkinson's disease. Stem Cells. 2014;32(8):2147-2163.[36] Lahti L, Peltopuro P, Piepponen TP, et al. Cell-autonomous FGF signaling regulates anteroposterior patterning and neuronal differentiation in the mesodiencephalic dopaminergic progenitor domain. Development. 2012;139(5):894-905.[37] Cooper O, Hargus G, Deleidi M, et al. Differentiation of human ES and Parkinson's disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol Cell Neurosci. 2010;45(3):258-266.[38] Chambers SM, Fasano CA, Papapetrou EP, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3): 275-280. ?[39] Morizane A, Doi D, Kikuchi T, et al. Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J Neurosci Res. 2011;89(2):117-126.[40] Cai J, Schleidt S, Pelta-Heller J, et al. BMP and TGF-β pathway mediators are critical upstream regulators of Wnt signaling during midbrain dopamine differentiation in human pluripotent stem cells. Dev Biol. 2013;376(1):62-73.[41] He XB, Kim M, Kim SY, et al. Vitamin C facilitates dopamine neuron differentiation in fetal midbrain through TET1- and JMJD3-dependent epigenetic control manner. Stem Cells. 2015;33(4):1320-1332.[42] Ning H, Huang YC, Banie L, et al. MicroRNA regulation of neuron-like differentiation of adipose tissue-derived stem cells. Differentiation. 2009;78(5):253-259.[43] Coyne L, Shan M, Przyborski SA, et al. Neuropharmacological properties of neurons derived from human stem cells. Neurochem Int. 2011;59(3):404-412.[44] Westerlund U, Moe MC, Varghese M, et al. Stem cells from the adult human brain develop into functional neurons in culture. Exp Cell Res. 2003;289(2):378-383.[45] Xi J, Liu Y, Liu H, et al. Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem Cells. 2012;30(8):1655-1663.[46] Stanslowsky N, Haase A, Martin U, et al. Functional differentiation of midbrain neurons from human cord blood-derived induced pluripotent stem cells. Stem Cell Res Ther. 2014;5(2):35.[47] Sagal J, Zhan X, Xu J, et al. Proneural transcription factor Atoh1 drives highly efficient differentiation of human pluripotent stem cells into dopaminergic neurons. Stem Cells Transl Med. 2014; 3(8):888-898. ?[48] Donato R, Miljan EA, Hines SJ, et al. Differential development of neuronal physiological responsiveness in two human neural stem cell lines. BMC Neurosci. 2007;8:36.[49] Ding S, Wei W, Zhou FM. Molecular and functional differences in voltage-activated sodium currents between GABA projection neurons and dopamine neurons in the substantia nigra. J Neurophysiol. 2011;106(6):3019-3034.[50] Seutin V, Engel D. Differences in Na+ conductance density and Na+ channel functional properties between dopamine and GABA neurons of the rat substantia nigra. J Neurophysiol. 2010;103(6):3099-3114.[51] Vazin T, Becker KG, Chen J, et al. A novel combination of factors, termed SPIE, which promotes dopaminergic neuron differentiation from human embryonic stem cells. PLoS One. 2009;4(8):e6606.[52] Schulz TC, Noggle SA, Palmarini GM, et al. Differentiation of human embryonic stem cells to dopaminergic neurons in serum-free suspension culture. Stem Cells. 2004;22(7): 1218-1238.[53] Hermann A, Maisel M, Wegner F, et al. Multipotent neural stem cells from the adult tegmentum with dopaminergic potential develop essential properties of functional neurons. Stem Cells. 2006;24(4):949-964. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Liu Feng, Peng Yuhuan, Luo Liangping, Wu Benqing. Plant-derived basic fibroblast growth factor maintains the growth and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1032-1037. |
| [4] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [5] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| [6] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [9] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [10] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [11] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [12] | Yang Sidi, Wang Qian, Xu Nuo, Wang Ronghan, Jin Chuanqi, Lu Ying, Dong Ming. Biodentine enhances the proliferation and differentiation of osteoblasts through upregulating bone morphogenetic protein-2 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 516-520. |
| [13] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [14] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [15] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||