Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (25): 3949-3955.doi: 10.3969/j.issn.2095-4344.2017.25.003
Previous Articles Next Articles
Genome-wide transcriptional profiling of NB4 leukemic cells affected by umbilical cord-derived mesenchymal stem cells
Fan Hui-fang, Chen Fang, Ma Feng-xia, Chi Ying, Lu Shi-hong, Han Zhong-chao
- State Key Laboratory of Experimental Hematology, Institute of Hematology and Hospital of Blood Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin 300020, China
-
Revised:2017-07-14Online:2017-09-08Published:2017-10-09 -
Contact:Han Zhong-chao, Professor, Doctoral supervisor, State Key Laboratory of Experimental Hematology, Institute of Hematology and Hospital of Blood Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin 300020, Chin -
About author:Fan Hui-fang, Studying for master′s degree, State Key Laboratory of Experimental Hematology, Institute of Hematology and Hospital of Blood Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin 300020, China -
Supported by:the National Natural Science Foundation of China, No. 81500098, 81330015; the CAMS Initiative for Innovative Medicine, No. 2016-I2M-1-017
CLC Number:
Cite this article
Fan Hui-fang, Chen Fang, Ma Feng-xia, Chi Ying, Lu Shi-hong, Han Zhong-chao. Genome-wide transcriptional profiling of NB4 leukemic cells affected by umbilical cord-derived mesenchymal stem cells[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(25): 3949-3955.
share this article
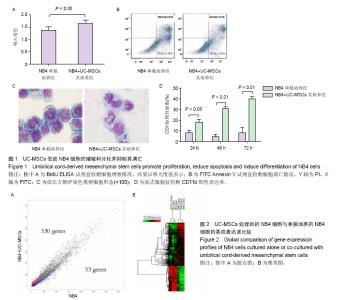
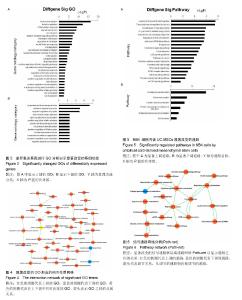
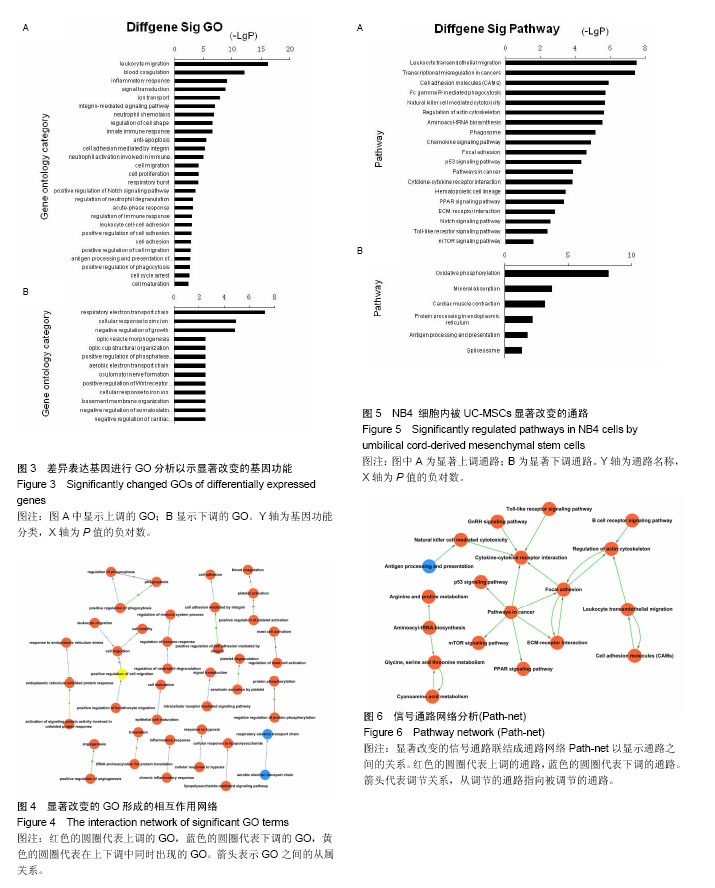
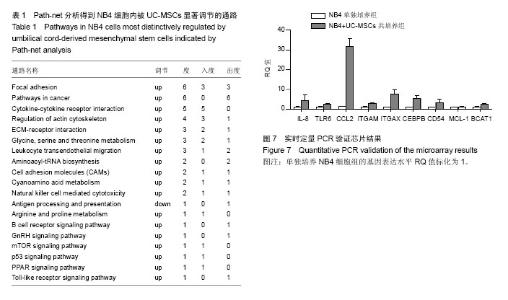
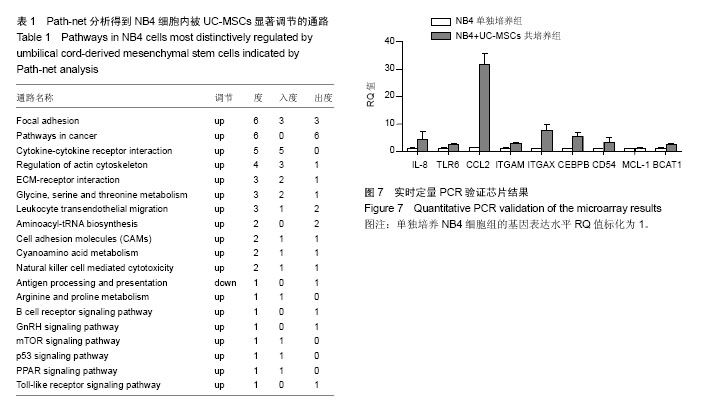
2.1 UC-MSCs影响NB4白血病细胞的生物学行为 共培养72 h后检测细胞增殖,UC-MSCs促进白血病细胞的生长(图1A);共培养48 h后检测细胞凋亡,UC-MSCs抑制5 μmol/L阿糖胞苷诱导的NB4细胞的凋亡(图1B);共培养72 h在显微镜下观察瑞士吉姆萨染色结果,并且于24,48,72 h分别检测细胞表面粒系分化抗原CD11b表达,NB4细胞出现了向粒细胞分化的特征(图1C和1D)。 2.2 通过随机方差模型(RVM)筛选差异基因 与NB4单独培养对照组相比,NB4+UC-MSCs共培养组的NB4细胞共有583个基因出现了明显的差异表达(P < 0.05,FDR < 0.05),其中530个基因上调,53个基因下调(图2A)。一些通常被认为与细胞增殖、凋亡、分化和免疫反应相关的基因如BCAT1,MCL-1,ITGAM,ITGAX,CEBPB,ICAM1,CD58,TLR4,TLR6,CCL2,IL-8等基因的表达分别增加了1.66倍,1.44倍,1.73倍,1.95倍,2.45倍,2.35倍,1.39倍,1.97倍,1.92倍,18.25倍和2.53倍(图2B)。 2.3 差异表达基因的GO分析 通过GO数据库对差异表达基因进行了GO分析,并通过双侧Fisher’s精确检验和χ2 检验评估数据的意义[13,18]。P < 0.01被认为差异有显著性意义。-LgP代表了调节显著程度。UC-MSCs作用下,上调最明显的5个GO分别为白细胞迁移(leukocyte migration),凝血功能(blood coagulation),炎症反应(inflammatory response),细胞信号转导(signal transduction)和离子转运(ion transport),见图3A。另外,整合素介导的信号通路(integrin-mediated signaling pathway),中性粒细胞趋化(neutrophil chemotaxis),细胞形态调节(regulation of cell shape),固有免疫应答(innate immune response),抗凋亡(anti-apoptosis),整合素介导的细胞黏附(cell adhesion mediated by integrin),免疫反应中中性粒细胞的激活(neutrophil activation involved in immune response),细胞增殖(cell proliferation),呼吸爆发(respiratory burst)和细胞成熟(cell maturation)相关的GO亦有明显上调。UC-MSCs作用下,下调的GO很少,下调最明显的5个GO分别是呼吸电子传递链(respiratory electron transport chain),细胞对锌离子的反应(cellular response to zinc ion),生长负调控(negative regulation of growth),视泡的形态发生(optic vesicle morphogenesis)和视杯的结构组织(optic cup structural organization),见图3B。 2.4 GO-Map分析 根据GO map能直接并系统地发现基因功能之间的网络关系。如图4所示,基因功能调节主要表现在4个方面:①免疫反应(immune response):免疫反应的调节(regulation of immune response),炎症反应(inflammatory response)和吞噬作用(phagocytosis);②细胞生理(Cellular process):细胞成熟(cell maturation)、迁移(cell migration)和黏附(cell adhesion);③对刺激的反应(response to stimuli):对低氧的反应(response to hypoxia),内质网应激(response to endoplasmic reticulum stress)和细胞对脂多糖的反应(cellular response to lipopolysaccharide);④细胞信号(cell signaling):信号转导(signal transduction)和蛋白磷酸化(protein phosphorylation)。 2.5 Pathway分析 根据KEGG数据库,分析了差异表达基因相关的信号通路,如图5所示,UC-MSCs作用下最有意义的通路是白细胞跨内皮迁移(leukocyte transendothelial migration),肿瘤转录失调(transcriptional misregulation in cancers),细胞黏附分子(CAMs),Fcγ受体介导的吞噬作用(Fc gamma-R mediated phagocytosis)和肌动蛋白骨架的调节(regulation of actin skeleton)。这些通路与中性粒细胞的激活关系密切,代表了中性粒细胞功能的成熟和形态的变化。 2.6 Path-Net分析 根据KEGG数据库进行了Path-Net分析。如图6所示,通路相互作用主要集中在斑状黏着(focal adhesion),肿瘤信号通路(pathways in cancer)和细胞因子-细胞因子受体相互作用(cytokine-cytokine receptor interaction)。另外还有氨基酸代谢网络(amino acid metabolism network)和肌动蛋白细胞骨架调节(regulation of actin cytoskeleton)网络。用“度(degree)”去评估通路的相互作用。表1列出了有意义的通路。斑状黏着(focal adhesion)(degree=6)和肿瘤信号通路(pathways in cancer)(degree=6)的degree最高,这预示着这两个信号通路在UC-MSCs对NB4细胞的影响上发挥了重要作用。 2.7 RT-PCR验证差异基因表达 选择数个功能密切相关的基因通过RT-PCR方法验证芯片分析的可信性。IL-8,TLR6,CCL2,ITGAM,ITGAX,CEBPB,CD54,MCL-1 和 BCAT1与主要的细胞生物学表现相关,对这几个基因进行了RT-PCR分析。基因芯片中上述基因的mRNA表达分别增加了2.53 倍,1.92倍,18.25倍,1.73倍,1.95倍,2.45倍,2.35倍,1.44倍和1.66 倍。RT-PCR数据与芯片结果一致,证实了与单独培养NB4细胞相比,这些基因在与UC-MSCs共培养组的NB4细胞中确实存在明显的上调(图7)。"

| [1] Kadiyala S, Young RG, Thiede MA, et al. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant. 1997;6(2): 125-134.[2] Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005; 106(5):1755-1761.[3] Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004; 363(9419):1439-1441.[4] Yu JM, Jun ES, Bae YC, et al. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev. 2008;17(3):463-473. [5] Zhu Y, Sun Z, Han Q, et al. Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia. 2009;23(5):925-933.[6] Ramasamy R, Lam EW, Soeiro I, et al. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21(2):304-310.[7] Khakoo AY, Pati S, Anderson SA, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006;203(5):1235-1247.[8] Dasari VR, Velpula KK, Kaur K, et al. Cord blood stem cell-mediated induction of apoptosis in glioma downregulates X-linked inhibitor of apoptosis protein (XIAP). PLoS One. 2010;5(7):e11813. [9] Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91(8):1017-1026.[10] Clarke R, Ressom HW, Wang A, et al. The properties of high-dimensional data spaces: implications for exploring gene and protein expression data. Nat Rev Cancer. 2008;8(1): 37-49.[11] Wright GW, Simon RM. A random variance model for detection of differential gene expression in small microarray experiments. Bioinformatics. 2003;19(18):2448-2455.[12] Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003;19(3):368-375.[13] Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25-29.[14] Draghici S, Khatri P, Tarca AL, et al. A systems biology approach for pathway level analysis. Genome Res. 2007; 17(10):1537-1545.[15] Kanehisa M, Goto S, Kawashima S, et al. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32(Database issue):D277-280.[16] Yi M, Horton JD, Cohen JC, et al. WholePathwayScope: a comprehensive pathway-based analysis tool for high-throughput data. BMC Bioinformatics. 2006;7:30.[17] Schlitt T, Palin K, Rung J, et al. From gene networks to gene function. Genome Res. 2003;13(12):2568-2576.[18] Dupuy D, Bertin N, Hidalgo CA, et al. Genome-scale analysis of in vivo spatiotemporal promoter activity in Caenorhabditis elegans. Nat Biotechnol. 2007;25(6):663-668.[19] Morley A, Higgs D. Lack of increased differentiation of leukemic cells in response to colony-stimulating factor. Cancer Res. 1974;34(9):2307-2310.[20] Morley A, Higgs D. Abnormal differentiation of leukemic cells in vitro. Cancer. 1974;33(3):716-720.[21] Moore MA, Williams N, Metcalf D. In vitro colony formation by normal and leukemic human hematopoietic cells: characterization of the colony-forming cells. J Natl Cancer Inst. 1973;50(3):603-623.[22] Marie JP, Izaguirre CA, Civin CI, et al. Granulopoietic differentiation in AML blasts in culture. Blood. 1981;58(4): 670-674.[23] Whittaker JA, Khurshid M, Hughes HR. Neutrophil function in chronic granulocytic leukaemia before and after busulphan treatment. Br J Haematol. 1974;28(4):541-549.[24] Rosner F, Valmont I, Kozinn PJ, et al. Leukocyte function in patients with leukemia. Cancer. 1970;25(4):835-842.[25] Yuan SY, Shen Q, Rigor RR, et al. Neutrophil transmigration, focal adhesion kinase and endothelial barrier function. Microvasc Res. 2012;83(1):82-88.[26] Patarroyo M, Makgoba MW. Leucocyte adhesion to cells in immune and inflammatory responses. Lancet. 1989;2(8672): 1139-1142.[27] Patarroyo M, Lindbom L. Leukocyte adhesion: a fundamental process in immunologic and inflammatory reactions. Lakartidningen. 1991;88(3):120-122.[28] Patarroyo M. Leukocyte adhesion in host defense and tissue injury. Clin Immunol Immunopathol. 1991;60(3):333-348. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [3] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [4] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [5] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [6] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [7] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [8] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [9] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [10] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [11] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [12] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [13] | Huang Chuanjun, Zou Yu, Zhou Xiaoting, Zhu Yangqing, Qian Wei, Zhang Wei, Liu Xing. Transplantation of umbilical cord mesenchymal stem cells encapsulated in RADA16-BDNF hydrogel promotes neurological recovery in an intracerebral hemorrhage rat model [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 510-515. |
| [14] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [15] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||