Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (13): 2087-2093.doi: 10.3969/j.issn.2095-4344.2017.13.020
Previous Articles Next Articles
Establishment and identification of pancreatic stem cell strain derived from islets of Kunming mice under feeder layer conditions
Cen Yan-hui1, 2, Yang Rui1, Jia Wei1, Li Zhong-hua1, Zhong Zhen-guo1, Zhong Jing1, Bao Juan1, He Guo-zhen1, Wu Xiao-jun1, Li Yi-yi1
- 1Basic Medical College, Guangxi University of Chinese Medicine, Nanning 530222, Guangxi Zhuang Autonomous Region, China; 2the First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning 530023, Guangxi Zhuang Autonomous Region, China
-
Revised:2017-03-22Online:2017-05-08Published:2017-06-09 -
Contact:Jia Wei, Master, Associate professor, Basic Medical College, Guangxi University of Chinese Medicine, Nanning 530222, Guangxi Zhuang Autonomous Region, China; Li Zhong-hua, Professor, Basic Medical College, Guangxi University of Chinese Medicine, Nanning 530222, Guangxi Zhuang Autonomous Region, China -
About author:Cen Yan-hui, M.D., Associate professor, Basic Medical College, Guangxi University of Chinese Medicine, Nanning 530222, Guangxi Zhuang Autonomous Region, China; the First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning 530023, Guangxi Zhuang Autonomous Region, China Yang Rui, Master, Basic Medical College, Guangxi University of Chinese Medicine, Nanning 530222, Guangxi Zhuang Autonomous Region, China Cen Yan-hui and Yang Rui contributed equally to this work. -
Supported by:the National Natural Science Foundation of China, No. 81503406; University Research Projects in Guangxi, No. ZD2014068; Project of Improving the Basic Ability of Young Teachers in Universities in Guangxi, No. KY2016YB218, KY2016YB224; Special Scientific Project of Traditional Chinese Medicine supported by Health Department of Guangxi Zhuang Autonomous Region, No. GZPT13-04, GZBZ16-07, GZLC16-23; and Student Research Training Project of Guangxi Medical University, No. 2014DXS02
CLC Number:
Cite this article
Cen Yan-hui, Yang Rui, Jia Wei, Li Zhong-hua, Zhong Zhen-guo, Zhong Jing, Bao Juan, He Guo-zhen, Wu Xiao-jun, Li Yi-yi. Establishment and identification of pancreatic stem cell strain derived from islets of Kunming mice under feeder layer conditions[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(13): 2087-2093.
share this article
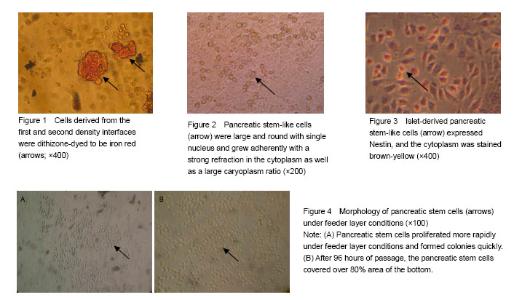
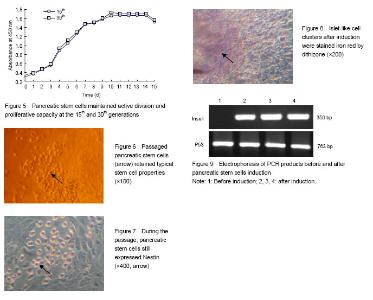
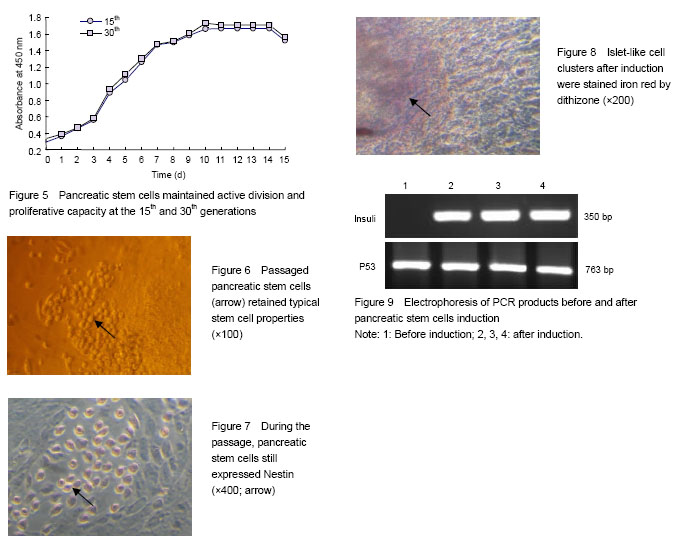
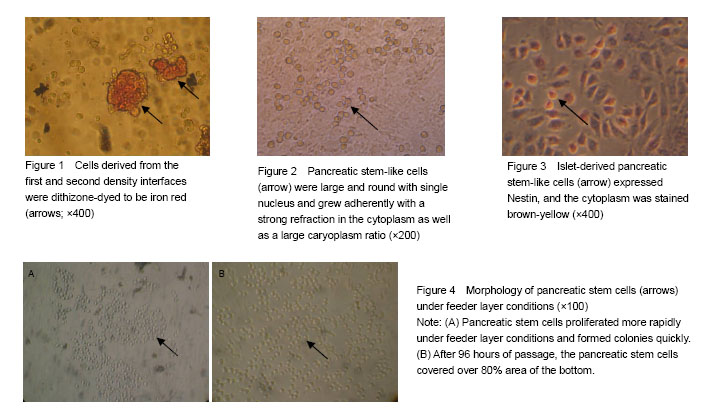
Identification of cell source after Percoll discontinuous density centrifugation fter Percoll discontinuous density centrifugation, the pancreatic tissue cells were distributed at three different densities (D-Hank’s/1.068, 1.068/1.096, 1.096/1.118) on the first, second and third density interfaces. Dithizone staining results showed that 80%-90% of the cells collected from the first and second density interfaces were iron red (Figure 1), indicating that the cells were derived from pancreatic endocrine portion (i.e., pancreas islet). Cells collected from the third density interface were non-stained with dithizone, indicating that the cells were derived from the exocrine portion of the pancreas. Primary culture and identification of pancreatic stem cells derived from pancreatic islets After 48 hours primary culture, from the pancreatic endocrine there were some large, round, and single-nucleated cells surrounding islet-like cell masses. These cells had strong cytoplasmic refraction, a large proportion of caryoplasm and grew adherently to the wall (Figure 2). These cells reflected the morphology characteristics of pancreatic stem cells, which were confirmed as pancreatic stem-like cells. The expression of Nestin in pancreatic stem cells was detected by immunocytochemical staining and got positive result as the cytoplasm was stained claybank. These confirmed the pancreatic stem-like cells were confirmed as islet-derived pancreatic stem cells (Figure 3). The pancreatic stem cells derived from pancreatic islets were continuously passaged to establish cell lines under feeder layer conditions The feeder layer was prepared from mouse embryonic desmocytes. Then, the pancreatic stem cells were subcultured in the feeder layer. After 48 hours of subculture, the pancreatic stem cells began to differentiate and proliferate and form large colonies (Figure 4A). After 96 hours of passage, the pancreatic stem cells could grow to more than 80% of the orifice area (Figure 4B). Then, the cells could be passaged continuously. Under stable in vitro conditions and with mature operation technique, pancreatic stem cells were passaged till the 30th generation to established the cell strain. Islet-derived pancreatic stem cell line detection Pancreatic stem cells in the process of continuous passages showed an active ability of division and proliferation (Figure 5). Pancreatic stem cell lines still maintained the original morphological features and expressed Nestin during the continuous passage. Under the microscope, the 15th and the 30th generations of pancreatic stem cells had large, round, single nucleus, large ratio of cytoplasm, and strong proliferating ability (Figure 6). Nestin staining showed positive results of all generations of pancreatic stem cells, while negative cells were found in the surrounding feeder layers (Figure 7). After continuous induction and differentiation, a part of cells began to gather, but no significant changes were found in cell morphology. These cells were divided to produce more cells budding to grow upward, forming a spherical islet-like cell structure after about 1 month, having spindly pedicles connected with the bottom of the bottle, and floating in the culture medium. Dithizone staining was iron red (Figure 8). The P53 gene of synthetic cDNA was amplified by RT-PCR, and the amplified band was about 763 bp by 1.0% agarose gel electrophoresis. This result indicated that the reverse transcription product cDNA had good quality. PCR was carried out using the specific primers of the insulin gene and the cDNA of the islet-like cell cluster as the template. The target band was found by 1.0% agarose gel electrophoresis. The molecular weight of PCR products was 50 bp in consistence with the expected molecular weight of the insulin gene (Figure 9). However, before induction, the cells did not express insulin mRNA."
| [1] Mathis D, Vence L, Benoist C. Beta-cell death during progression to diabetes. Nature. 2001;414(6865):792-798. [2] China has become the most serious diabetes country in the world, and the incidence in children is doubled every 10 years. Shanghai Yufang Yixue. 2010;22(6):340.[3] Berna G, leon-Quinto T, Ensenat-Waser T, et al. Stem cells and diabetes. Biomed Pharmacothe. 2001;55:206- 212. [4] Ye RG. Internal Medicine (5th edition). Beijing: People's Medical Publishing House. 2002.[5] Rosenberg L, Vinik AI, Pittenger GL, et al. Islet cell regeneration in the diabetic hamster pancreas with restoration of normoglycaemia can be induced by a local growth factor(s). Diabetologia. 1996;39:256-262. [6] Bonner-Weir S, Taneja M, Weir GC, et al. Differentiantion of islet cells in long-term. Pancrea. 2000;20:337-347. [7] Song ZS, Gu KJ. Study on transdifferentiation of adult pancreatic stem cells into islets. Zhonghua Waike Zazhi. 2002;40(11):807-810.[8] Xiao M. Human pancreatic stem cell line establishment and its induction of islet transplantation to treat diabetes of rats. Xianyang: Northwest Sci-Tech University of Agriculture and Forestry.[9] Mckinnon VM, Docherty K. Pancreatic duodenal homeobox-1, pdx-1, a major regulator of beta cell identity and function. Diabetologia. 2001;44:1203-1214. [10] Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin–promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606-609.[11] Offield MF, Jetton TL, Labosky PA, et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983-995. [12] He JD. Differentiation of rabbit bone marrow mesenchymal stem cells into pancreatic islet cells. Zhengzhou: Henan Agricultural University. 2011.[13] Jing H, Li Z, Lin X, et al. The role of human ADA2a in the regulation of p53 acetylation and stability. Zhongguo Kexue Tongbao. 2011;56(4):397-405.[14] Güre AO, Stockert E, Arden KC, et al. CT10: a new cancer-testis (CT) antigen homologous to CT7 and the MAGE family, identified by representational-difference analysis. Int J Cancer. 2000;85(5):726-732. [15] Palm K, Salin-Nordstrom T, Levesque MF, et al. Fetal and adult human CNS stem cells have similar molecular characteristics and developmental potential. Dev Brain Res. 2000;78:192-195. [16] Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585-595. [17] Dahlstrand J, Zimmerman LB, McKay RD, et al. Characterization of the human nestin gene reveals a close evolutionary relationship to neurofilaments. Cell Sci. 1992; 103:589-597.[18] Hunziker E, Stein M. Nestin-expressing cells in the pancreatic islets of Langerhans. Biochem Biophys Res Commun. 2000;271(1):116-119.[19] Zulewski H, Abraham EJ, Gerlach MU, et al. Multipotential nestin positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50(3): 521-533.[20] Lumelsky N, Blondel O, Laeng P, et al. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292(5520): 1389- 1394.[21] Abraham EJ, Leech CA, Lin JC, et al. Insulinotropic hormone glucagon-like peptide-1 differentiation of human pancreatic islet-derived progenitor cells into insulin- producing cells. Endocrinology. 2002;143:3152-3161.[22] Blyszczuk P, Czyz J, Kania G, et al. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin positive progenitor and insulin-producing cells. Proc Natl Acad Sci USA. 2003;100:998-1003. [23] Evans MJ, Kaufman MH. Establishment in culture of pluripotential from mouse embryos. Nature. 1981; 292(5819):154-156. |
| [1] | Gao Lei, Qin Xinyuan, Nie Xin, Wang Lei, Wang Jiangning. Extracorporeal circulation compression perfusion in the reconstruction of limb microcirculation from the mechanism of mechanical and chemical signal transduction [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1334-1340. |
| [2] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [3] | Lü Yiyan, Li Hanbing, Ma Xiaoqing, Zhang Han, Zhang Yuhang, Li Genlin. Establishment and characteristic analysis of interior heat and diabetes mouse model using compound factors [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1187-1193. |
| [4] | Chen Xianghe, Liu Bo, Yang Kang, Lu Pengcheng, Yu Huilin. Treadmill exercise improves the myocardial fibrosis of spontaneous type 2 diabetic mice: an exploration on the functional pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1210-1215. |
| [5] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [6] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [7] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [8] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [9] | Luo Xiaoling, Zhang Li, Yang Maohua, Xu Jie, Xu Xiaomei. Effect of naringenin on osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1051-1056. |
| [10] | Wang Xinmin, Liu Fei, Xu Jie, Bai Yuxi, Lü Jian. Core decompression combined with dental pulp stem cells in the treatment of steroid-associated femoral head necrosis in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1074-1079. |
| [11] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [12] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [13] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [14] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| [15] | Hui Xiaoshan, Bai Jing, Zhou Siyuan, Wang Jie, Zhang Jinsheng, He Qingyong, Meng Peipei. Theoretical mechanism of traditional Chinese medicine theory on stem cell induced differentiation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1125-1129. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||