Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (6): 962-968.doi: 10.3969/j.issn.2095-4344.2017.06.025
Previous Articles Next Articles
Peripheral nerve injury and regeneration: application and progress of novel nerve scaffolds
- 1Orthopedic Institute of General Hospital of Chinese PLA, Beijing Key Lab of Regenerative Medicine, Key Laboratory of Trauma & War Injuries of Chinese PLA, Beijing 100853, China; 2Department of Orthopaedics, the First Affiliated Hospital of General Hospital of Chinese PLA, Beijing 100048, China
-
Received:2016-12-06Online:2017-02-28Published:2017-03-16 -
Contact:Peng Jiang, Associate researcher, Orthopedic Institute of General Hospital of Chinese PLA, Beijing Key Lab of Regenerative Medicine, Key Laboratory of Trauma & War Injuries of Chinese PLA, Beijing 100853, China Zhao Qing, Chief physician, Professor, Department of Orthopaedics, the First Affiliated Hospital of General Hospital of Chinese PLA, Beijing 100048, China -
About author:Quan Qi, Studying for doctorate, Orthopedic Institute of General Hospital of Chinese PLA, Beijing Key Lab of Regenerative Medicine, Key Laboratory of Trauma & War Injuries of Chinese PLA, Beijing 100853, China -
Supported by:the National Natural Science Foundation of China, No. 51073024, 51273021; 973 key Program of China, No. 2014CB542201, 2012CB518106; the Special Project of the “Thirteenth Five-year Plan” for Medicine Development of Chinese PLA, No. BWS13C029; the Translational Medicine Project of the 301st Hospital of PLA, No. 2016TM-030; the Major Research and Development Project of China during the Thirteenth Five-Year Period, No. 2016YFC1101600
CLC Number:
Cite this article
Quan Qi, Chang Biao, Liu Ruo-xi, Sun Xun, Wang Yu, Lu Shi-bi, Peng Jiang, Zhao Qing.
share this article
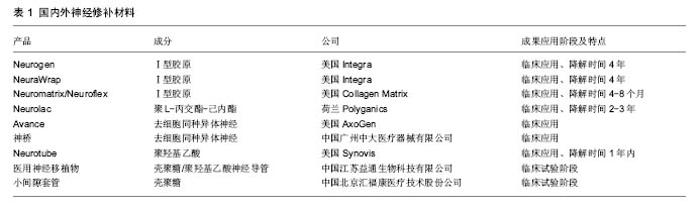
2.1 周围神经损伤与再生 2.1.1 瓦勒氏变性 周围神经损伤,通常是指轴突损伤,轴突是神经元的巨大细胞结构,单维持其本身正常生理功能就是一个挑战[3]。当其受到如挤压、撕裂、甚至完全断裂等损伤后的几分钟内,其末端便开始形成营养不良性小泡,这种早期的变性即为急性轴突损伤[4],当损伤变得不可逆的时,残端轴突将会在24-72 h发生崩解,之后便是许旺细胞的增殖与巨噬细胞的清理这一不可逆的过程。这一现象在1850年被Augustus Volney Waller发现并描述,也就是人们既熟悉又陌生的瓦勒氏变性。熟悉是因为距这一概念的提出已有165年了,陌生是因为时至今日,对远端轴突发生的瓦勒氏变性的机制仍然不清晰[5],遥想十几年前,人们还简单的认为瓦勒变性是由于缺乏来自胞体的营养,而发生的营养不良性变性,直到Wlds的出现,才让人们意识到,缺乏营养的远端轴突在一些状态下也可保持基本形态长达数周[6-7]。Wlds的发现,对于轴突的认识从传统的组织学、生理学、药理学研究拓展到分子生物学与生物信息学[8]。急性轴突退变被认为在损伤开始的几分钟内发生,这一过程包括第二信使的改变、细胞骨架结构的变化、程序化死亡的开启(凋亡)。过去认为在损伤的那一刻,钙离子的内流与分布改变,诱导了细胞骨架的变化,不能维持正常结构的轴突只能发生瓦勒氏变性[9]。而近期研究表明,钙离子或许不是早期的瓦勒氏变性的起始点[10]。作者通过对Schmidt- Lanterman clefts这一轴突微结构的周围钙离子分布进行研究,发现在轴突损伤的前4 h内,钙离子分布并无显著变化。而这4 h也是明显长于通常定义的急性轴突退变时限。 细胞的自噬并不是单纯意义上的死亡,或电镜下出现吞噬泡这一碗型结构,自噬对于细胞起着双重作用,一方面,自噬在清除降解异常蛋白,恢复正常细胞代谢和功能上有保护作用;另一方面,如果这种反应过大,达到一定阈值后,细胞器便会开始损伤,造成对细胞的伤害,这一过程中的主要受害者便是有着能量工厂之称的线粒体。线粒体在神经轴突中如何担负起供能任务,曾经一度被认为是解决瓦勒氏变性的关键一环。因为ATP在细胞浆中有限的扩散能力(特别对于轴突来说,这简直是万里长征),线粒体—可移动的能量工厂—对于保障突触末端递质释放时瞬时大量ATP的消耗就显得至关重要。那瓦勒氏变性重要原因,是因为线粒体在轴突运输的障碍吗?正常情况下,线粒体在轴突中通过Syntaphilin的锚定作用,及时准确地转移到需要部位,静态情况下线粒体常固定在最需要能量的部位,如突触前膜,正常状态下参与线粒体固定运输的酶有很多,如Klf5、trak1、trak2等,其中以Klf5最为人所熟知[11]。Klf5分为A/B/C三型,在神经特异性表达的是A/C型,如果阻滞其作用,可以观测到的线粒体密度分布异常,慢性退行性轴突病变被证明与Kif5a相关[12]。在一些少见的人类相关疾病中,由Kif5Aa突变所导致的线粒体密度改变,常导致轴突的变性[13],这一变性除了许旺细胞与巨噬细胞迁移、增殖、分化外,其余病理改变与瓦勒氏变性类似[14]。但这一过程往往是需要较长时间完成的,相较于定义4 h左右的急性轴突退变还是明显滞后许多,现有的实验数据不支持线粒体分布异常在急性期引发了瓦勒氏变性。但就其是否导致了远端轴突突触结构功能的丧失,还不明朗。可由于线粒体分布密度的巨大变化,确实可导致轴突变性,究其根本,似乎可归因于能量供应出现的问题。 2.1.2 由WLDs得出新的认识 当发现WLDs小鼠后,改变了人们一些固有的思考。其一,相较于正常情况下损伤远端轴突基本结构维持1到2 d,在WLDs模型中,残端轴突可维持几周[7]。这一发现使科学家意识到,在一些情况下,作为一个巨大细胞结构的轴突,即使缺少胞体的调控,也可维持很长的一段时间。其二,缺少胞体的支持,在能量及营养缺乏的情况下,轴突在相当长的一段时间内也可维持正常的形态与功能[15]。其三,与其说瓦勒氏变性是一个被动的日渐降解的病理过程,不如说是一个类似于凋亡,主动衰减的过程[16-17]。Wlds不仅可以在哺乳动物中发挥作用,也在以果蝇或斑马鱼的实验模型中发挥作用[6,18]。Wlds由Nmnat1和ube4b两段基因编码,其中Nmnat主要发挥抗瓦勒氏变性的作用[19]。而ube4b由N16与N70组成,则被认为与Wlds作用位点有关[20]。Nmnat1是一种核内的NAD+合成酶,但仅仅Nmnat1的单独作用不能阻止瓦勒氏变性[21-22]。虽然目前大家仍然没有找到阻止瓦勒氏变性的根本方法,但基于现有研究,认为瓦勒氏变性是与轴突能量供应紧密相关的,最近的研究表明SARM1是激活轴突变性的起点[23-24]。 大量研究表明,调控急性与慢性轴突变性的机制没有明显区别,也就是说,在减少轴突变性上,急性与慢性神经损伤上的策略很可能是一致的[19,25]。以往认为损伤后的轴突坏死归因于胞体能量供应的不足,但通过对损伤后轴突细胞自噬机制的研究,发现也许轴突自身的调节发挥着更为重要的作用[26],而这种影响并不单来自胞体营养的缺乏[27]。通过对周围神经瓦勒氏变性机制更加深入的研究,也许可使人们接近神经焊接这一梦想,但现阶段还言之过早。 2.1.3 Bungner氏带的形成与轴突再生 神经细胞轴突的再生与上述过程同时发生,由许旺细胞与巨噬细胞组成的“清道夫”开始进行“打扫”工作[28]。提别是许旺细胞还同时形成Bungner氏带。这条由许旺细胞形成的高速通道连接了损伤神经的两端[29]。为了方便理解,神经的再生可以分为5个时期[30],水肿渗出期,基质形成期,细胞迁移期,轴突再生期,髓鞘形成期。简单的概括周围神经轴突再生的特点:损伤的神经组织渗出富含细胞外基质及神经营养因子的渗出液,一般损伤后3-6 h渗出达峰。第一、二期形成重要的纤维索,之后再生的许旺细胞沿着纤维索形成Bungner氏带[31],轴突的再生随之开始,与此同时,再生型许旺细胞开始分化,形成成熟的髓鞘型许旺细胞,在轴突到达远端神经支配区域后,纤维索便开始降解,最后再生的轴突外形成髓鞘[2,32]。 2.1.4 周围神经轴突再生过程中的关键细胞 许旺细胞起着支持并诱导再生轴突的重要功能[33]。由许旺细胞构建的再生微环境[34],不仅对新生的轴突起着力学支撑的作用[29],并且由其分泌的多种细胞因子,包括神经生长因子、脑源性神经生长因子、胰岛素样因子,均诱导并促进了轴突的再生[35-36]。干细胞如诱导多能干细胞[37]、神经干细胞、间充质干细胞等均被证明可促进神经的再生[38-39],其中以诱导多能干细胞最为引人瞩目[40-41],其在中枢神经与周围神经中均得到了成功运用。并且,由诱导多能干细胞诱导形成的许旺细胞生理学功能与天然环境下的许旺细胞相似。但复合干细胞的支架也面临着巨大挑战,如干细胞植入人工支架后,不受控制的增殖与分化[42];耦合细胞的支架在植入体内后引起异常疼痛等问题[43]。 支架复合许旺细胞:用于定植于支架上的许旺细胞可分为同基因型与非同基因型,无论是哪种,都可提高轴突的再生[28]。但总体来讲同基因型来源的许旺细胞修复效果优于异基因型,这并不难理解,同基因型,代表着更少的炎症反应、无排斥反应、与再生过程中的其他细胞配合默契。而对于非同基因型的则需要免疫抑制剂来保障植入细胞的存活,常用的免疫抑制剂有经典的环孢素A,他克莫司、JNJ460(FK506的衍生物)。实验表明,免疫抑制剂的介入有利于异基因型许旺细胞发挥功能,并且有助于周围神经再生[36,44-45]。同基因型许旺细胞来源有限,准备周期时间长,保存困难,这些限制都是广泛应用的巨大障碍。还有少部分许旺细胞可来自许旺细胞系,有证据表明这种来源的细胞也表现出令人满意的效果[46]。确定来源的许旺细胞可直接与支架共培养,与神经修补材料形成整体,丰富修补材料的功能[47]。 2.2 新型神经修补支架 2.2.1 材料 自体神经移植一直是治疗大段神经缺损的金标准,但这一治疗方法并不完美,存在着供体区域感觉功能丧失、来源有限、运动与感觉不匹配及瘢痕等问题[30, 48-50]。因此科学家从未放弃探索新型的神经修补材料以取代自体神经移植。长段神经缺损的修复与再生存在着两大难题。第一,神经修补不是简单的“修补”,而是要通过修补材料架起的“桥梁”引导近端神经纤维快速长向靶器官,减少因失神经引起的靶器官萎缩;第二,绝大部分的周围神经是感觉与运动的混合支,因此须使感觉、运动神经纤维准确到达相应靶器官,才能准确实现神经再支配。简而言之,新型神经修补材料的构建主要集中在使再生神经长得“快”与长得“准”这两点上。 神经修补材料或神经修复导管,距今已有100多年的历史了,从1882年空心骨质导管成功修复犬坐骨神经缺损,到如今复杂内部空间结构并添加天然神经诱导因子的复合导管的研制[51],大段神经缺损修复走过了激动人心的100年。随着人们对神经再生基础研究的深入,发现空心修复导管很难满足神经功能恢复的要求[52]。表1总结了国内外部分神经导管产品[49,53-55]。 基础医学新的发现,引导着人们发展具有更加复杂内部空间结构的新型导管。理想的神经移植物应该具有如下特点:良好的生物相容性及温和的降解过程,避免引起炎性反应,已有实验证实,炎性反应不利于神经再生;管壁允许营养物质与细胞因子的交换,内壁可进行修饰或复合二级空间结构,达到形态仿生;释放与神经再生相关的生长因子;便于临床操作。 根据神经导管的材料来源可分为天然与合成材料两类[56]。天然材料包括自体静脉、自体骨骼肌、异体或异种神经、藻酸盐、胶原[57]、壳聚糖等[58];人工合成材料主要有硅胶、聚乳酸-羟基乙酸共聚物、聚乳酸等[59]。静脉、骨骼肌等天然生物活性材料存在管壁塌陷、瘢痕组织增生及粘连等问题且来源均比较有限,不适于大批量生产。人工合成材料主要是制备成中空管状结构的引导再生导管,但这种导管的缺点主要是渗透性差,无取向性,不利于近端轴突的生长。构建神经导管的方法包括化学去细胞法[60]、静电纺丝法[61]、3D打印、相分离技术等。应明确的是,这两大类材料间可相互混合,取长补短,形成复合材料,改善如亲水性在内的多种物理化学特性,如壳聚糖与聚羟基乙酸的混合就被证明有减轻炎症反应,促进血管生成的作用[62]。 支架结构与形状:亚里士多德曾说过“形状是生命的灵魂”。支架结构和形状的设计,决定着支架的性能,从最简单的单纯管状结构到复杂的多管道且内部纤维排列方向与神经生长方向一致都影响着神经的再生。 单纯管状结构:应用如电纺丝法制备管状模型的方法大体可有两类,应用模具方法或单纯应用薄膜通过卷曲的方式制造[33]。模具法可选用可溶型或不可溶型的模具。可溶型微管道模型材料选取可溶于水的有机材料,如聚乙烯醇、蚕丝蛋白/聚环氧乙烷、聚乙二醇等,依材料熔点不同选取特定温度,熔融状态下制成不同直径圆柱状模型,取出时溶于水中,得到管状神经支架。对于不溶型的模具可选用金属模具,模具外涂抹如聚乙二醇等物质,制备完成后,溶于水中,易于取下。 复合型神经支架:为了更好地模拟天然神经结构,科学家在中空管道内引入了呈取向排列的纤维,试图模拟损伤神经在修复再生过程中纤维索的形成,引导许旺细胞的迁移、增殖及分泌形成细胞外基质,加速神经再生过程。纤维的材料可以是聚L-丙交酯、聚乳酸-羟基乙酸共聚物、聚乙醇酸等高分子聚合物,也可以是壳聚糖、蚕丝等天然材料[63]。在一项为期6个月的研究里,应用聚乳酸-羟基乙酸共聚物纤维与间充质干细胞修复犬50 mm坐骨神经损伤,动物神经功能的恢复明显高于低密度时,神经修复能力得到增强[65-66]。 2.2.3 国内在这一领域的进展 近年来国内科学工作者在周围神经再生领域也取得了令人欣喜的成功。中山大学刘小林教授团队研发了全球第二个去细胞神经移植物(神桥),目前已在全国90余家医院使用,取得了良好临床效果[67]。南通大学顾晓松院士、杨宇民教授团队发明的壳聚糖中和固定加工技术,制备壳聚糖/PGA神经移植物,率先将壳聚糖人工神经移植物应用于临床,并成功实现了35 mm长段周围神经缺损的修复,Science杂志曾专门刊文报道,后期研究在神经导管中添加蚕丝,引导再生轴突取向排列也取得了成功[54,62]。北京大学的姜保国教授、张殿英教授团队研发的体内可降解生物套管,在小间隙套接修复神经损伤修复实验中被证明优于传统神经外膜缝合技术,恒河猴实验更表明植入材料取得了优秀的修复效果[68]。由武汉理工大学戴红莲教授团队研发的仿生纳米复合可降解神经移植物,重塑神经再生过程中的纳米微环境,促进了NGF、BDNF的合成与表达,被证实可诱导神经再生[69-70]。解放军第四军医大学罗卓荆教授团队研发的导电神经修补材料,模拟了生理条件下生物电对再生轴突的刺激,体外实验表明有利于轴突再生[35]。"

| [1]Coleman M.Axon degeneration mechanisms: commonality amid diversity.Nat Rev Neurosci.2005;6:889-898.[2]Deumens R,Bozkurt A,Meek MF,et al.Repairing injured peripheral nerves: Bridging the gap.Prog Neurobiol. 2010; 92:245-276.[3]Coleman MP,Freeman MR.Wallerian degeneration, wld(s), and nmnat.Annu Rev Neurosci.2010;33:245-267.[4]Rotshenker S.Wallerian degeneration: the innate-immune response to traumatic nerve injury.J Neuroinflammation. 2011;8:109.[5]Raff MC,Whitmore AV,Finn JT.Axonal self-destruction and neurodegeneration. Science.2002;296:868-871.[6]Meyerzu Horste G,Miesbach TA,Muller JI,et al.The Wlds transgene reduces axon loss in a Charcot-Marie-Tooth disease 1A rat model and nicotinamide delays post-traumatic axonal degeneration.Neurobiol Dis.2011;42:1-8.[7]Avery MA,Rooney TM,Pandya JD,et al.WldS prevents axon degeneration through increased mitochondrial flux and enhanced mitochondrial Ca2+ buffering.Curr Biol. 2012;22: 596-600.[8]Wang JT,Barres BA.Axon degeneration: where the Wlds things are.Curr Biol. 2012;22:R221-223.[9]Vargas ME,Barres BA.Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci.2007;30:153-179.[10]Adalbert R,Morreale G,Paizs M,et al.Intra-axonal calcium changes after axotomy in wild-type and slow Wallerian degeneration axons.Neuroscience. 2012;225:44-54.[11]Hirokawa N,Noda Y,Tanaka Y,et al.Kinesin superfamily motor proteins and intracellular transport.Nature reviews Molecular cell biology.2009;10:682-696.[12]Karle KN,Mockel D,Reid E,et al.Axonal transport deficit in a KIF5A( -/- ) mouse model.Neurogenetics.2012;13:169-179.[13]Kawaguchi K.Role of kinesin-1 in the pathogenesis of SPG10, a rare form of hereditary spastic paraplegia. Neuroscientist. 2013;19:336-344.[14]Hinckelmann MV,Zala D,Saudou F.Releasing the brake: restoring fast axonal transport in neurodegenerative disorders.Trends Cell Biol.2013;23:634-643.[15]O'Donnell KC,Vargas ME,Sagasti A.WldS and PGC-1alpha regulate mitochondrial transport and oxidation state after axonal injury.J Neurosci.2013;33:14778-14790.[16]Freeman MR.Signaling mechanisms regulating Wallerian degeneration.Curr Opin Neurobiol.2014;27:224-231.[17]Wang JT,Medress ZA,Barres BA.Axon degeneration: Molecular mechanisms of a self-destruction pathway.JCell Biol.2012;196:7-18.[18]Coleman MP.The challenges of axon survival: introduction to the special issue on axonal degeneration.Exp Neurol.2013; 246:1-5.[19]Mack TG,Reiner M,Beirowski B,et al.Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene.Nat Neurosci.2001;4:1199-1206.[20]Brown R,Hynes-Allen A,Swan AJ,et al.Activity-dependent degeneration of axotomized neuromuscular synapses in Wld S mice.Neuroscience.2015;290:300-320.[21]Avery MA,Sheehan AE,Kerr KS,et al.WldS requires Nmnat1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration.J Cell Biol.2009;184:501-513.[22]Gilley J,Coleman MP.Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons.PLoS Biol. 2010;8:e1000300.[23]Gerdts J,Brace EJ,Sasaki Y,et al.SARM1 activation triggers axon degeneration locally via NAD(+) destruction. Science. 2015;348:453-457.[24]Osterloh JM,Yang J,Rooney TM,et al.dSarm/Sarm1 is required for activation of an injury-induced axon death pathway.Science.2012;337:481-484.[25]Knoferle J,Koch JC,Ostendorf T,et al.Mechanisms of acute axonal degeneration in the optic nerve in vivo.Proc Natl Acad Sci U S A.2010;107:6064-6069.[26]Franze K,Janmey PA,Guck J.Mechanics in neuronal development and repair. Annu Rev Biomed Eng.2013;15: 227-251.[27]Cashman CR,Hoke A.Mechanisms of distal axonal degeneration in peripheral neuropathies.Neurosci Lett.2015; 596:33-50.[28]Mosahebi A,Fuller P,Wiberg M,et al.Effect of allogeneic Schwann cell transplantation on peripheral nerve regeneration.Exp Neurol.2002;173:213-223.[29]Arthur-Farraj PJ,Latouche M,Wilton DK,et al.c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration.Neuron. 2012;75: 633-647.[30]Daly W,Yao L,Zeugolis D,et al.A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface. 2012;9:202-221.[31]Kalbermatten DF,Kingham PJ,Mahay D,et al.Fibrin matrix for suspension of regenerative cells in an artificial nerve conduit.J Plast Reconstr Aesthet Surg. 2008;61:669-675.[32]Pettersson J,Kalbermatten D,McGrath A,et al.Biodegradable fibrin conduit promotes long-term regeneration after peripheral nerve injury in adult rats.J Plast Reconstr Aesthet Surg.2010;63:1893-1899.[33]Wang W,Itoh S,Konno K,et al.Effects of Schwann cell alignment along the oriented electrospun chitosan nanofibers on nerve regeneration.J Biomed Mater Res A. 2009;91: 994-1005.[34]Chew SY,Mi R,Hoke A,et al.The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation. Biomaterials.2008;29:653-661.[35]Huang J,Hu X,Lu L,et al.Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers.J Biomed Mater Res A.2010;93:164-174.[36]Wang X,Xu XM.Long-term survival, axonal growth-promotion, and myelination of Schwann cells grafted into contused spinal cord in adult rats.Exp Neurol. 2014;261:308-319.[37]Uemura T,Takamatsu K,Ikeda M,et al.Transplantation of induced pluripotent stem cell-derived neurospheres for peripheral nerve repair.Biochem Biophys Res Commun. 2012; 419:130-135.[38]Ruckh TT,Kumar K,Kipper MJ,et al.Osteogenic differentiation of bone marrow stromal cells on poly(epsilon-caprolactone) nanofiber scaffolds.Acta Biomater. 2010;6:2949-2959.[39]Zhou LN,Zhang JW,Liu XL,et al.Co-Graft of Bone Marrow Stromal Cells and Schwann Cells Into Acellular Nerve Scaffold for Sciatic Nerve Regeneration in Rats. J Oral Maxillofac Surg.2015;73(8):1651-1660.[40]Haastert-Talini K,Grothe C.Comment to the paper: Acceleration of peripheral nerve regeneration using nerve conduits in combination with induced pluripotent stem cell technology and a basic fibroblast growth factor drug delivery system by M. Ikeda, T. Uemura, K. Takamatsu, M. Okada, K. Kazuki, Y. Tabata, Y. Ikada, H. Nakamura, J Biomed Mater Res A. 2013 Jun 3 doi: 10.1002/jbm.a.34816.J Biomed Mater Res A.2014;102:1219-1220.[41]Ribeiro J,Pereira T,Caseiro AR,et al.Evaluation of biodegradable electric conductive tube-guides and mesenchymal stem cells.World J Stem Cells.2015;7:956-975.[42]Levenberg S,Huang NF,Lavik E,et al.Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci U S A.2003;100:12741-12746.[43]Hofstetter CP,Holmstrom NA,Lilja JA,et al.Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8(3): 346-353.[44]Birge RB,Wadsworth S,Akakura R,et al.A role for schwann cells in the neuroregenerative effects of a non-immunosuppressive fk506 derivative, jnj460. Neuroscience.2004;124:351-366.[45]McGrath AM,Brohlin M,Kingham PJ,et al.Fibrin conduit supplemented with human mesenchymal stem cells and immunosuppressive treatment enhances regeneration after peripheral nerve injury.Neurosci Lett.2012;516:171-176.[46]Lawrence JM,Keegan DJ,Muir EM,et al.Transplantation of Schwann cell line clones secreting GDNF or BDNF into the retinas of dystrophic Royal College of Surgeons rats.Invest Ophthalmol Vis Sci.2004;45:267-274.[47]Valmikinathan CM,Hoffman J,Yu X.Impact of Scaffold Micro and Macro Architecture on Schwann Cell Proliferation under Dynamic Conditions in a Rotating Wall Vessel Bioreactor.Mater Sci Eng C Mater Biol Appl.2011;31:22-29.[48]Isaacs J.Treatment of acute peripheral nerve injuries: current concepts.J Hand Surg Am.2010;35:491-497.[49]Pabari A,Yang SY,Seifalian AM,et al.Modern surgical management of peripheral nerve gap. J Plast Reconstr Aesthet Surg.2010;63:1941-1948.[50]Bellamkonda RV.Peripheral nerve regeneration: an opinion on channels, scaffolds and anisotropy.Biomaterials. 2006; 27:3515-3518.[51]Dinis TM,Elia R,Vidal G,et al.3D multi-channel bi-functionalized silk electrospun conduits for peripheral nerve regeneration.J Mech Behav Biomed Mater. 2015;41:43-55.[52]Ribeiro-Resende VT,Koenig B,Nichterwitz S,et al.Strategies for inducing the formation of bands of Bungner in peripheral nerveregeneration.Biomaterials.2009;30:5251-5259.[53]Griffin J,Carbone A,Delgado-Rivera R,et al.Design and evaluation of novel polyanhydride blends as nerve guidance conduits.Acta Biomater.2010;6:1917-1924.[54]Gu X,Ding F,Yang Y,et al.Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration.Prog Neurobiol.2011;93:204-230.[55]Bai L,Wang TB,Wang X,et al.Use of nerve elongator to repair short-distance peripheral nerve defects: a prospective randomized study.Neural Regen Res.2015;10:79-83.[56]Araujo JV,Carvalho PP,Best SM.Electrospinning of Bioinspired Polymer Scaffolds.Adv Exp Med Biol. 2015;881: 33-53.[57]Liu T,Teng WK,Chan BP,et al.Photochemical crosslinked electrospun collagen nanofibers: synthesis, characterization and neural stem cell interactions.J Biomed Mater Res A. 2010; 95:276-282.[58]Wang W,Itoh S,Matsuda A,et al.Enhanced nerve regeneration through a bilayered chitosan tube: the effect of introduction of glycine spacer into the CYIGSR sequence.J Biomed Mater Res A.2008;85:919-928.[59]Panseri S,Cunha C,Lowery J,et al.Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections.BMC Biotechnol.2008;8:39.[60]Hu J,Zhu QT,Liu XL,et al.Repair of extended peripheral nerve lesions in rhesus monkeys using acellular allogenic nerve grafts implanted with autologous mesenchymal stem cells. Exp Neurol.2007;204:658-666.[61]Prabhakaran MP,Venugopal JR,Chyan TT,et al.Electrospun biocomposite nanofibrous scaffolds for neural tissue engineering. Tissue Eng Part A. 2008;14:1787-1797.[62]Gu Y,Zhu J,Xue C,et al.Chitosan/silk fibroin-based, Schwann cell-derived extracellular matrix-modified scaffolds for bridging rat sciatic nerve gaps. Biomaterials. 2014;35: 2253-2263.[63]Rutkowski GE,Miller CA,Jeftinija S,et al.Synergistic effects of micropatterned biodegradable conduits and Schwann cells on sciatic nerve regeneration.J Neural Eng.2004;1:151-157.[64]Ding F,Wu J,Yang Y,et al.Use of tissue-engineered nerve grafts consisting of a chitosan/poly(lactic-co-glycolic acid)-based scaffold included with bone marrow mesenchymal cells for bridging 50-mm dog sciatic nerve gaps.Tissue Eng Part A. 2010;16:3779-3790.[65]Ngo TT,Waggoner PJ,Romero AA,et al.Poly(L-Lactide) microfilaments enhance peripheral nerve regeneration across extended nerve lesions.J Neurosci Res.2003;72:227-238.[66]Stang F,Fansa H,Wolf G,et al.Structural parameters of collagen nerve grafts influence peripheral nerve regeneration. Biomaterials.2005;26:3083-3091.[67]Zhu S,Liu J,Zheng C,et al.Analysis of human acellular nerve allograft reconstruction of 64 injured nerves in the hand and upper extremity: a 3 year follow-up study.J Tissue Eng Regen Med.2016.doi: 10.1002/term.2130.[Epub ahead of print][68]Zhang PX,Li-Ya A,Kou YH,et al.Biological conduit small gap sleeve bridging method for peripheral nerve injury: regeneration law of nerve fibers in the conduit.Neural Regen Res.2015;10(1):71-78.[69]Yi J,Xiong F,Li B,et al.Degradation characteristics, cell viability and host tissue responses of PDLLA-based scaffold with PRGD and beta-TCP nanoparticles incorporation.Regen Biomater.2016;3(3):159-166.[70]Qiu T,Yin Y,Li B,et al.PDLLA/PRGD/beta-TCP conduits build the neurotrophin-rich microenvironment suppressing the oxidative stress and promoting the sciatic nerve regeneration.J Biomed Mater Res A.2014;102:3734-3743.[71]Jha BS,Colello RJ,Bowman JR,et al.Two pole air gap electrospinning: Fabrication of highly aligned, three-dimensional scaffolds for nerve reconstruction.Acta Biomater.2011;7:203-215.[72]Hoffmann N,Mittnacht U,Hartmann H,et al.Neuronal and glial responses to siRNA-coated nerve guide implants in vitro. Neurosci Lett.2011;494:14-18.[73]Ghasemi-Mobarakeh L,Prabhakaran MP,Morshed M,et al. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering.J Tissue Eng Regen Med.2011;5:e17-35.[74]Prabhakaran MP,Ghasemi-Mobarakeh L,Jin G,et al. Electrospun conducting polymer nanofibers and electrical stimulation of nerve stem cells.J Biosci Bioeng. 2011;112(5): 501-507.[75]Tavangarian F,Li Y.Carbon nanostructures as nerve scaffolds for repairing large gaps in severed nerves.CeramInt. 2012;38: 6075-6090.[76]Cattin AL,Burden JJ,Van Emmenis L,et al. Macrophage- Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves.Cell. 2015; 162: 1127-1139. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [3] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [4] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [5] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [6] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [9] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [10] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [11] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [12] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [13] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| [14] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| [15] | Li Xuan, Sun Yimin, Li Longbiao, Wang Zhenming, Yang Jing, Wang Chenglin, Ye Ling. Manufacturing of nano-modified polycaprolactone microspheres and its biological effects in dental pulp cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1530-1536. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||