Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (24): 3634-3641.doi: 10.3969/j.issn.2095-4344.2016.24.021
Previous Articles Next Articles
Regulation of osteoarthritis hypoxia-inducible factor-2 alpha regulatory mechanism and application prospect
Yang Chao1, Zhang Lei2, Zhou Li-wu2, Zhao Jian-ning2
- 1Department of Orthopedics, Clinical Institute of Nanjing Medical University, Nanjing 210000, Jiangsu Province, China; 2Department of Orthopedics, Nanjing General Hospital of Nanjing Military Command, Nanjing 210002, Jiangsu Province, China
-
Online:2016-06-10Published:2016-06-10 -
Contact:Zhao Jian-ning, Chief physician, Doctoral supervisor, Department of Orthopedics, Nanjing General Hospital of Nanjing Military Command, Nanjing 210002, Jiangsu Province, China -
About author:Yang Chao, Studying for master’s degree, Department of Orthopedics, Clinical Institute of Nanjing Medical University, Nanjing 210000, Jiangsu Province,China Zhang Lei, M.D., Attending physician, Department of Orthopedics, Nanjing General Hospital of Nanjing Military Command, Nanjing 210002, Jiangsu Province, China Yang Chao and Zhang Lei contributed equally to this work. -
Supported by:the Medical Scientific Research Foundation of Jiangsu Province, No. BL2012002
CLC Number:
Cite this article
Yang Chao, Zhang Lei, Zhou Li-wu, Zhao Jian-ning. Regulation of osteoarthritis hypoxia-inducible factor-2 alpha regulatory mechanism and application prospect[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(24): 3634-3641.
share this article
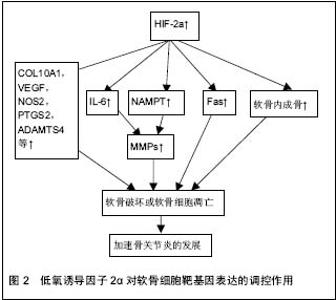
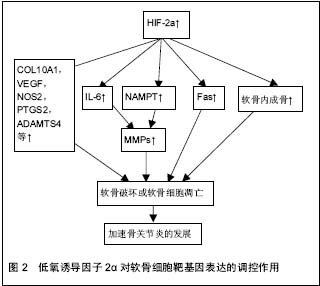
2.1 低氧诱导因子的结构 低氧诱导因子属于转录因子家族,低氧诱导因子家族有α-和β-两个亚基组成。α亚基对氧敏感,主要分为低氧诱导因子1α、低氧诱导因子2α和低氧诱导因子3α,其中低氧诱导因子1α和低氧诱导因子2α是细胞对氧敏感的关键效应器;β亚基又称为芳烃受体核转位因子(ARNT),在细胞内稳定表达[3-4]。在常温下,α亚基残端的脯氨酸(P402和P564)和天冬酰胺(N803)相应地被低氧诱导因子脯氨酸羟化酶和HIF抑制因子所羟基化,脯氨酸羟基化 诱发pVHL蛋白介导的泛素化,并促使α亚基在蛋白酶体中降解。然而,在低氧条件下,低氧诱导因子α脯氨酸羟基化受阻,pVHL所介导的泛素化-蛋白酶体受到抑制,导致α-亚基聚集并转入到细胞核内,与β-亚基结合形成异二聚体,从而激发下游的靶基因[5]。 2.2 低氧诱导因子对关节软骨细胞的作用 关节软骨是一种无血管、神经分布的组织,软骨细胞主要从周围的关节滑液以及软骨下骨中摄取营养和氧,因此关节软骨细胞终身处于这种低氧的环境之中。正是由于软骨细胞这种的内在性质-缺乏神经分布和血管供应,导致成熟的软骨自我修复能力极低。关节软骨由两种主要的细胞外基质大分子构成:Ⅱ型胶原和蛋白聚糖。软骨细胞的退变坏死与骨关节炎的发生发展密切相关,骨关节炎中软骨细胞的退变坏死不仅与细胞外基质的流失有关,而且与软骨细胞的凋亡有关。 低氧诱导因子1α在正常人和骨关节炎患者的软骨细胞中表达,因此软骨细胞中低氧诱导因子1α的表达增加可能是软骨处于低氧环境中的一种适应性改变。低氧诱导因子1α在软骨细胞分化的早期阶段表达,是机体在低氧环境下对体内氧平衡的关键调控因子;然而,低氧诱导因子2α于软骨细胞分化的后期阶段表达,在软骨退变以及软骨内成骨中扮演重要的角色。在软骨的组织工程研究中,低氧诱导因子1α表现出良好的软骨形成能力。在人骨髓细胞中过表达低氧诱导因子1α,甚至不需要外在的生长因子就能有效的诱导出软骨细胞[6]。在骨关节炎患者以及健康的人和大鼠的软骨中,低氧诱导因子2α的表达同样是增强的[5,7]。有研究表明,在低氧环境中,造血干细胞的移植效率以及低氧诱导因子的表达都显著提高[8]。低氧诱导因子1α和低氧诱导因子2α的分布和功能各不相同,在关节软骨及软骨细胞中的作用甚至相反[9]。研究表明低氧诱导因子1α不仅可以在基因水平调控SOX9、Ⅱ型胶原、蛋白聚糖的表达,并且可以同时抑制COL1A1、COL1A2和COL3A1的表达,从而促进软骨的形成;还可以调控软骨细胞的自噬和细胞凋亡,并在细胞的无氧酵解中起着重要的作用。因此,低氧诱导因子1α可能是通过促进软骨细胞形成,维持软骨细胞活性,促使软骨细胞适应低氧环境,从而来保护关节软骨[10]。 相比于低氧诱导因子1α在软骨细胞中扮演一个保护性的角色,低氧诱导因子2α在软骨细胞中更像是一个分解代谢基因的调控者。低氧诱导因子2α似乎并不是通过直接调控细胞外基质的合成或者软骨细胞的细胞调亡作用于软骨细胞,而是通过调控软骨细胞靶基因的表达,扮演一个分解调控因子的角色(见图2)。低氧诱导因子2α是由Epas1基因编码,主要表达在软骨周边钙化层高分化的肥大软骨细胞中,诱导软骨凋亡[11]。低氧诱导因子2α是关节软骨破坏的调控者,通过调控各种分解代谢基因的表达来引发骨关节炎患者的软骨破坏,其中包括基质分解酶类和各种炎性因子等。所以目前认为低氧诱导因子2α可能与骨关节炎的发生发展以及软骨细胞的破坏关系更加密切。低氧诱导因子2α在骨关节炎患者以及骨关节炎模型大鼠的软骨细胞中显著地增加,Epas1转基因的小鼠在软骨细胞中表现出自发性的软骨破坏,然而Epas1基因缺失的小鼠则表现出抑制软骨破坏[12]。因此,通过调控关节软骨细胞中Epas1基因的表达,有针对性地抑制关节软骨的破坏,可能是一种可行的治疗骨关节炎的策略。 尽管对不同亚型的低氧诱导因子在骨关节炎中的具体作用机制仍在探索中,但目前普遍认为低氧诱导因子1α对关节软骨主要起着保护作用;而低氧诱导因子2α则是关节软骨破坏的调控者,不同亚型的低氧诱导因子对软骨细胞的调控机制各不相同。所以只有明确了各亚型的低氧诱导因子在骨关节炎中不同的作用机制,以及低氧诱导因子对生理状态及病理状态下的关节软骨的不同影响,才能做到有选择的、明确的利用不同亚型的低氧诱导因子来调控软骨细胞的合成及分解代谢,最终达到促进关节软骨愈合的目的。"
| [1] Neogi T.Clinical significance of bone changes in osteoarthritis. Ther Adv Musculoskelet Dis.2012;4(4): 259-267. [2] 余扬,崔磊.膝骨性关节炎软骨组织低氧诱导因子1α和粒细胞-巨噬细胞集落刺激因子的表达[J]. 中国组织工程研究, 2015(11):1683-1687. [3] Saito T,Kawaguchi H.HIF-2alpha as a possible therapeutic target of osteoarthritis. Osteoarthritis Cartilage.2010;18(12):1552-1556. [4] Semenza GL, Prabhakar NR.The role of hypoxia-inducible factors in oxygen sensing by the carotid body. Adv Exp Med Biol.2012;758:1-5. [5] Saito T, Fukai A, Mabuchi A, et al.Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat Med.2010;16(6):678-686. [6] Duval E, Baugé C, Andriamanalijaona R,et al.Molecular mechanism of hypoxia-induced chondrogenesis and its application in in vivo cartilage tissue engineering. Biomaterials.2012;33(26):6042-6051. [7] Semenza GL.Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399-408. [8] Mantel CR, O'Leary HA, Chitteti BR,et al.Enhancing Hematopoietic Stem Cell Transplantation Efficacy by Mitigating Oxygen Shock. Cell.2015;161(7): 1553-1565. [9] Wu L, Huang X, Li L, Huang H, et al.Insights on Biology and Pathology of HIF-1 alpha/-2 alpha, TGF beta/BMP, Wnt/beta-Catenin, and NF-kappa B Pathways in Osteoarthritis. Current Pharmaceutical Design.2012;18(22):3293-3312. [10] Zhang FJ, Luo W, Lei GH.Role of HIF-1alpha and HIF-2alpha in osteoarthritis. Joint Bone Spine.2015; 82(3): 144-147. [11] Patel SA,Simon MC.Biology of hypoxia-inducible factor-2alpha in development and disease. Cell Death Differ.2008;15(4): 628-634. [12] Yang S, Kim J, Ryu JH, et al.Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med.2010;16(6): 687-693. [13] Wang M, Shen J, Jin H, et al.Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Skeletal Biology and Medicine Ii: Bone and Cartilage Homeostasis and Bone Disease.2011;1240:61-69. [14] Premaraj S, Souza I, Premaraj T.Mechanical loading activates beta-catenin signaling in periodontal ligament cells. Angle Orthod.2011;81(4): 592-599. [15] Choi H, Chun YS, Kim TY, et al.HIF-2alpha enhances beta-catenin/TCF-driven transcription by interacting with beta-catenin. Cancer Res.2010;s0(24): p. 10101-10111. [16] Yang Y. Wnts and wing: Wnt signaling in vertebrate limb development and musculoskeletal morphogenesis. Birth Defects Res C Embryo Today.2003;69(4): 305-317. [17] 刘丰,彭昊,周建林,等.骨关节炎关节软骨细胞中低氧诱导因子2α及血管内皮生长因子的表达[J].中国组织工程研究, 2015,19(2): 201-206. [18] Pi Y, Zhang X, Shao Z,et al.Intra-articular delivery of anti-Hif-2 alpha siRNA by chondrocyte-homing nanoparticles to prevent cartilage degeneration in arthritic mice. Gene Ther.2015;22(6):439-448. [19] Higgs R.Osteoarthritis: HIF-2alpha drives osteoarthritis development. Nat Rev Rheumatol.2010;6(8): 435. [20] Inoue H, Arai Y, Kishida T, et al.Hydrostatic pressure influences HIF-2 alpha expression in chondrocytes. Int J Mol Sci.2015;16(1): 1043-1050. [21] Ryu JH, Yang S, Shin Y,et al.Interleukin-6 plays an essential role in hypoxia-inducible factor 2alpha-induced experimental osteoarthritic cartilage destruction in mice. Arthritis Rheum.2011; 63(9): 2732-2743. [22] Chang J, Jackson SG, Wardale J, et al.Hypoxia modulates the phenotype of osteoblasts isolated from knee osteoarthritis patients, leading to undermineralized bone nodule formation. Arthritis Rheumatol.2014;66(7): 1789-1799. [23] Hirata M, Kugimiya F, Fukai A,et al.C/EBP beta and RUNX2 cooperate to degrade cartilage with MMP-13 as the target and HIF-2 alpha as the inducer in chondrocytes. Human Molecular Genetics.2012; 21(5):1111-1123. [24] Ding M, Lu Y, Abbassi S, et al.Targeting Runx2 expression in hypertrophic chondrocytes impairs endochondral ossification during early skeletal development. Journal of Cellular Physiology. 2012; 227(10):3446-3456. [25] Gao SG, Li KH, Zeng KB, et al.Elevated osteopontin level of synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis patients. Osteoarthritis and Cartilage.2010;18(1):82-87. [26] Cheng C, Zhang FJ, Tian J, et al.Osteopontin inhibits HIF-2 alpha mRNA expression in osteoarthritic chondrocytes. Experimental and Therapeutic Medicine, 2015. 9(6): 2415-2419. [27] Weng T, Xie Y, Yi L,et al.Loss of Vhl in cartilage accelerated the progression of age-associated and surgically induced murine osteoarthritis. Osteoarthritis and Cartilage.2014;22(8):1197-1205. [28] Yang X, Yang ZJ, Liu FX, et al.Inhibition of mTOR and HIF pathways diminishes chondro-osteogenesis and cell proliferation in chondroblastoma. Tumour Biol. 2013;34(5):3111-3119. [29] Bohensky J, Terkhorn SP, Freeman TA,et al.Regulation of Autophagy in Human and Murine Cartilage Hypoxia-Inducible Factor 2 Suppresses Chondrocyte Autophagy. Arthritis and Rheumatism.2009;60(5): 1406-1415. [30] Duan Y, Hao D, Li M, et al.Increased synovial fluid visfatin is positively linked to cartilage degradation biomarkers in osteoarthritis.Rheumatology Int.2012; 32(4):985-990. [31] Hong EH, Yun HS, Kim J, et al.Nicotinamide Phosphoribosyltransferase Is Essential for Interleukin-1 beta-mediated Dedifferentiation of Articular Chondrocytes via SIRT1 and Extracellular Signal-regulated Kinase (ERK) Complex Signaling. J Biol Chem. 2011;286(32):28619-28631. [32] Yang S, Ryu JH, Oh H, et al.NAMPT (visfatin), a direct target of hypoxia-inducible factor-2 alpha, is an essential catabolic regulator of osteoarthritis. Annals of the Rheumatic Diseases.2015;74(3):595-602. [33] Oh H, Kwak JS, Yang S, et al.Reciprocal regulation by hypoxia-inducible factor-2alpha and the NAMPT-NAD-SIRT axis in articular chondrocytes is involved in osteoarthritis. Osteoarthritis Cartilage. 2015; 23(12):2288-2296. [34] Kim HA, Blanco FJ.Cell death and apoptosis in osteoarthritic cartilage. Curr Drug Targets.2007; 8(2): 333-345. [35] Ryu JH, Shin Y, Huh YH, et al.Hypoxia-inducible factor-2 alpha regulates Fas-mediated chondrocyte apoptosis during osteoarthritic cartilage destruction. Cell Death and Differentiation.2012; 19(3): 440-450. [36] Kim JH, Jeon J, Shin M, et al.Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8- MTF1 axis. Cell.2014; 156(4):730-743. [37] Lee M, Won Y, Shin Y, et al.Reciprocal activation of hypoxia-inducible factor (HIF)-2alpha and the zinc-ZIP8-MTF1 axis amplifies catabolic signaling in osteoarthritis. Osteoarthritis Cartilage. 2016 ;24(1): 134-145. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Peng Zhihao, Feng Zongquan, Zou Yonggen, Niu Guoqing, Wu Feng. Relationship of lower limb force line and the progression of lateral compartment arthritis after unicompartmental knee arthroplasty with mobile bearing [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1368-1374. |
| [3] | Huang Dengcheng, Wang Zhike, Cao Xuewei. Comparison of the short-term efficacy of extracorporeal shock wave therapy for middle-aged and elderly knee osteoarthritis: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1471-1476. |
| [4] | Liu Xiangxiang, Huang Yunmei, Chen Wenlie, Lin Ruhui, Lu Xiaodong, Li Zuanfang, Xu Yaye, Huang Meiya, Li Xihai. Ultrastructural changes of the white zone cells of the meniscus in a rat model of early osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1237-1242. |
| [5] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [6] | Liu Xin, Yan Feihua, Hong Kunhao. Delaying cartilage degeneration by regulating the expression of aquaporins in rats with knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 668-673. |
| [7] | Ma Zetao, Zeng Hui, Wang Deli, Weng Jian, Feng Song. MicroRNA-138-5p regulates chondrocyte proliferation and autophagy [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 674-678. |
| [8] | Cao Xuhan, Bai Zixing, Sun Chengyi, Yang Yanjun, Sun Weidong. Mechanism of “Ruxiang-Moyao” herbal pair in the treatment of knee osteoarthritis based on network pharmacology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 746-753. |
| [9] | Li Yonghua, Feng Qiang, Tan Renting, Huang Shifu, Qiu Jinlong, Yin Heng. Molecular mechanism of Eucommia ulmoides active ingredients treating synovitis of knee osteoarthritis: an analysis based on network pharmacology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 765-771. |
| [10] | Song Shan, Hu Fangyuan, Qiao Jun, Wang Jia, Zhang Shengxiao, Li Xiaofeng. An insight into biomarkers of osteoarthritis synovium based on bioinformatics [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 785-790. |
| [11] | Deng Zhenhan, Huang Yong, Xiao Lulu, Chen Yulin, Zhu Weimin, Lu Wei, Wang Daping. Role and application of bone morphogenetic proteins in articular cartilage regeneration [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 798-806. |
| [12] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [13] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [14] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [15] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||