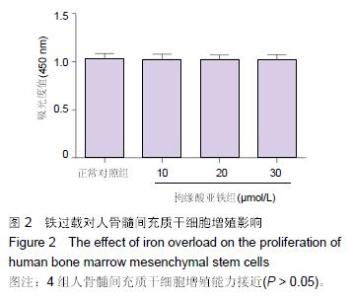
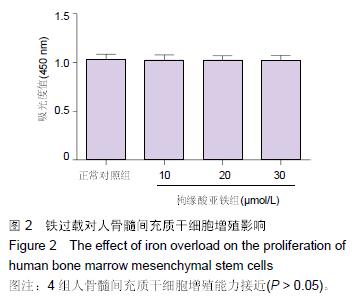
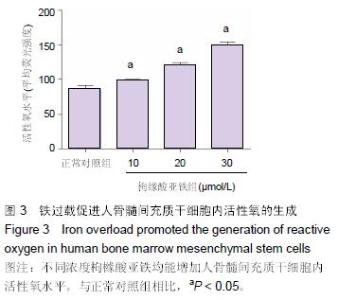
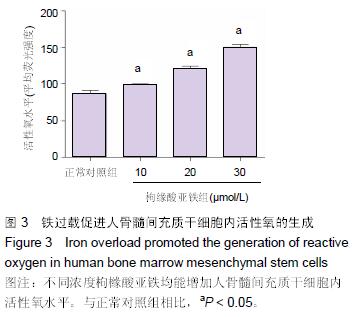
Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (14): 2007-2014.doi: 10.3969/j.issn.2095-4344.2016.14.004
Previous Articles Next Articles
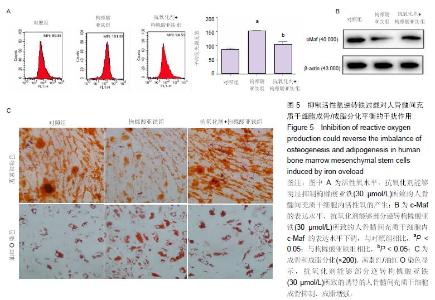
Iron overload inhibits osteogenesis and promotes adipogenesis in human bone marrow mesenchymal stem cells by producing reactive oxygen
Han Yan-jiu, Liu Guo-hui, Liu Yong
- Department of Orthopedics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China
-
Received:2016-03-01Online:2016-04-01Published:2016-04-01 -
Contact:Liu Guo-hui, Doctoral supervisor, Professor, Chief physician, Department of Orthopedics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China -
About author:Han Yan-jiu, Studying for doctorate, Attending physician, Department of Orthopedics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China -
Supported by:the National Natural Science Foundation of China, No. 30973045
Cite this article
Han Yan-jiu, Liu Guo-hui, Liu Yong. Iron overload inhibits osteogenesis and promotes adipogenesis in human bone marrow mesenchymal stem cells by producing reactive oxygen[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(14): 2007-2014.
share this article
| [1] Domrongkitchaiporn S, Sirikulchayanonta V, Angchaisuksiri P, et al. Abnormalities in bone mineral density and bone histology in thalassemia. J Bone Miner Res. 2003;18(9):1682-1688. [2] Terpos E, Voskaridou E. Treatment options for thalassemia patients with osteoporosis. Ann N Y Acad Sci. 2010;1202:237-243. [3] Mahachoklertwattana P, Sirikulchayanonta V, Chuansumrit A, et al. Bone histomorphometry in children and adolescents with beta-thalassemia disease: iron-associated focal osteomalacia. J Clin Endocrinol Metab. 2003;88(8):3966-3972. [4] Doyard M, Chappard D, Leroyer P, et al. Decreased bone formation explains osteoporosis in a genetic mouse model of hemochromatosiss. PLoS One. 2016; 11(2):e0148292. [5] Kim BJ, Ahn SH, Bae SJ, et al. Iron overload accelerates bone loss in healthy postmenopausal women and middle-aged men: a 3-yearretrospective longitudinal study. J Bone Miner Res. 2012;27(11): 2279-2290. [6] Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014; 10(1):9-17. [7] Tsay J, Yang Z, Ross FP, et al. Bone loss caused by iron overload in a murine model: importance of oxidative stress. Blood. 2010;116(14):2582-2589. [8] Czekanska EM, Stoddart MJ, Richards RG, et al. In search of an osteoblast cell model for in vitro research. Eur Cell Mater. 2012;24:1-17. [9] 田青,刘勇,李国庆,等.老年骨质疏松骨折血清铁水平的测定及临床意义[J].临床急诊杂志,2013,(3):10-16. [10] Messer JG, Kilbarger AK, Erikson KM, et al. Iron overload alters iron-regulatory genes and proteins, down-regulates osteoblastic phenotype, and is associated with apoptosis in fetal rat calvaria cultures. Bone. 2009;45(5):972-979. [11] Li GF, Pan YZ, Sirois P, et al. Iron homeostasis in osteoporosis and its clinical implications. Osteoporos Int. 2012;23(10):2403-2408. [12] Vogiatzi MG, Macklin EA, Fung EB, et al. Bone disease in thalassemia: a frequent and still unresolved problem. J Bone Miner Res. 2009;24(3):543-557. [13] Yang Q, Jian J, Abramson SB, et al. Inhibitory effects of iron on bone morphogenetic protein 2-induced osteobl- astogenesis. J Bone Miner Res. 2011;26(6):1188-1196. [14] Guan M, Yao W, Liu R, et al. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med. 2012;18(3):456-462. [15] Nishikawa K, Nakashima T, Takeda S, et al. Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation. J Clin Invest. 2010;120(10):3455-3465. [16] Veronesi F, Torricelli P, Borsari V, et al. Mesenchymal stem cells in the aging and osteoporotic population. Crit Rev Eukaryot Gene Expr. 2011;21(4):363-377. [17] Singh L, Brennan TA1, Russell E1, et al. Aging alters bone-fat reciprocity by shifting in vivo mesenchymal precursor cell fate towards an adipogenic lineage. Bone. 2016;85:29-36. [18] Shen J, James AW, Zhang X, et al. Novel Wnt regulator NEL-Like molecule-1 antagonizes adipogenesis and augments osteogenesis induced by bone morphogenetic protein 2. Am J Pathol. 2016;186(2):419-434. [19] Ge C, Cawthorn WP, Li Y, et al. Reciprocal control of osteogenic and adipogenic differentiation by ERK/MAP kinase phosphorylation of Runx2 and PPARγ transcri- ption factors. J Cell Physiol. 2016;231(3): 587-596. [20] Tang L, Chen Y, Pei F, et al. Lithium chloride modulates adipogenesis and osteogenesis of human bone marrow-derived mesenchymal stem cells. Cell Physiol Biochem. 2015;37(1):143-152. [21] Chen Q, Shou P, Zheng C, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016. in press. [22] Li P, Yang YM, Sanchez S, et al. Deubiquitinase MYSM1 is essential for normal bone formation and mesenchymal stem cell differentiation. Sci Rep. 2016;6:22211. [23] Chen PM, Lin CH, Li NT, et al. c-Maf regulates pluripotency genes, proliferation/self-renewal, and lineage commitment in ROS-mediated senescence of human mesenchymal stem cells. Oncotarget. 2015; 6(34):35404-35418. [24] Saidak Z, Haÿ E, Marty C, et al. Strontium ranelate rebalances bone marrow adipogenesis and osteoblastogenesis in senescent osteopenic mice through NFATc/Maf and Wnt signaling. Aging Cell. 2012;11(3):467-474. [25] Gennari L, Rotatori S, Bianciardi S, et al. Treatment needs and current options for postmenopausal osteoporosis. Expert Opin Pharmacother. 2016. in press. [26] Karasik D, Rivadeneira F, Johnson ML. The genetics of bone mass and susceptibility to bone diseases. Nat Rev Rheumatol. 2016. in press. [27] Gibon E, Lu L, Goodman SB. Aging, inflammation, stem cells, and bone healing. Stem Cell Res Ther. 2016;7(1):44. [28] Loi F, Córdova LA, Pajarinen J, et al. Inflammation, fracture and bone repair. Bone. 2016;86:119-130. [29] Lindsay R, Krege JH, Marin F, et al. Teriparatide for osteoporosis: importance of the full course. Osteoporos Int. 2016. in press. [30] Black DM, Rosen CJ. Clinical Practice. Postmenopausal Osteoporosis. N Engl J Med. 2016 ;374(3):254-262. [31] Li GF, Pan YZ, Sirois P, et al. Iron homeostasis in osteoporosis and its clinical implications. Osteoporos Int. 2012;23(10):2403-2408. [32] Zhao L, Wang Y, Wang Z, et al. Effects of dietary resveratrol on excess-iron-induced bone loss via antioxidative character. J Nutr Biochem. 2015;26(11): 1174-1182. [33] Chen B, Li GF, Shen Y, et al. Reducing iron accumulation: A potential approach for the prevention and treatment of postmenopausal osteoporosis. Exp Ther Med. 2015;10(1):7-11. [34] Xiao W, Beibei F, Guangsi S, et al. Iron overload increases osteoclastogenesis and aggravates the effects of ovariectomy on bone mass. J Endocrinol. 2015;226(3):121-134. [35] Tournis S, Dede AD, Savvidis C, et al. Effects of teriparatide retreatment in a patient with β-thalassemia major. Transfusion. 2015;55(12):2905-2910. [36] Wong P, Polkinghorne K, Kerr PG, et al. Deferasirox at therapeutic doses is associated with dose-dependent hypercalciuria. Bone. 2016;85:55-58. [37] Rossi F, Perrotta S, Bellini G, et al. Iron overload causes osteoporosis in thalassemia major patients through interaction with transient receptor potential vanilloid type 1 (TRPV1) channels. Haematologica. 2014;99(12):1876-1884. [38] Chen B, Yan YL, Liu C, et al. Therapeutic effect of deferoxamine on iron overload-induced inhibition of osteogenesis in a zebrafish model. Calcif Tissue Int. 2014;94(3):353-360. [39] Zhou J, Ye S, Fujiwara T, et al. Steap4 plays a critical role in osteoclastogenesis in vitro by regulating cellular iron/reactive oxygen species (ROS) levels and cAMP response element-binding protein (CREB) activation. J Biol Chem. 2013;288(42):30064-30074.
[40] Wong P, Fuller PJ, Gillespie MT, et al. Thalassemia bone disease: the association between nephrolithiasis, bone mineral density and fractures. Osteoporos Int. 2013;24(7):1965-1971. [41] Kim MK, Lee JW, Baek KH, et al. Endocrinopathies in transfusion-associated iron overload. Clin Endocrinol (Oxf). 2013;78(2):271-277. [42] Lu W, Zhao M, Rajbhandary S, et al. Free iron catalyzes oxidative damage to hematopoietic cells/mesenchymal stem cells in vitro and suppresses hematopoiesis in iron overload patients. Eur J Haematol. 2013;91(3):249-261. [43] Zhao GY, Zhao LP, He YF, et al. A comparison of the biological activities of human osteoblast hFOB1.19 between iron excess and iron deficiency. Biol Trace Elem Res. 2012;150(1-3):487-495. [44] Messer JG, Kilbarger AK, Erikson KM, et al. Iron overload alters iron-regulatory genes and proteins, down-regulates osteoblastic phenotype, and is associated with apoptosis in fetal rat calvaria cultures. Bone. 2009;45(5):972-979. [45] Singh L, Brennan TA, Russell E, et al. Aging alters bone-fat reciprocity by shifting in vivo mesenchymal precursor cell fate towards an adipogenic lineage. Bone. 2016;85:29-36. [46] Zhang Y, Ma C, Liu X, et al. Epigenetic landscape in PPARγ2 in the enhancement of adipogenesis of mouse osteoporotic bone marrow stromal cell. Biochim Biophys Acta. 2015;1852(11):2504-2516. [47] Simann M, Schneider V, Le Blanc S, et al. Heparin affects human bone marrow stromal cell fate: Promoting osteogenic and reducing adipogenic differentiation and conversion. Bone. 2015;78:102-113. [48] Watt J, Schlezinger JJ. Structurally-diverse, PPARγ-activating environmental toxicants induce adipogenesis and suppress osteogenesis in bone marrow mesenchymal stromal cells. Toxicology. 2015;331:66-77. [49] Li CJ, Cheng P, Liang MK, et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Invest. 2015;125(4): 1509-1522. [50] Tan XL, Zhang YH, Cai JP, et al. 5-(Hydroxymethyl)-2-furaldehyde inhibits adipogenic and enhances osteogenic differentiation of rat bone mesenchymal stem cells. Nat Prod Commun. 2014; 9(4):529-532. [51] Lee DS, Choung HW, Kim HJ, et al. NFI-C regulates osteoblast differentiation via control of osterix expression. Stem Cells. 2014;32(9):2467-2479. [52] Soucie EL, Weng Z, Geirsdóttir L, et al. Lineage-specific enhancers activate self-renewal genes in macrophages and embryonic stem cells. Science. 2016;351(6274):aad5510. [53] Pellegrino S, Ronda L, Annoni C, et al. Molecular insights into dimerization inhibition of c-Maf transcription factor. Biochim Biophys Acta. 2014; 1844(12):2108-2115. [54] Wende H, Lechner SG, Cheret C, et al. The transcription factor c-Maf controls touch receptor development and function. Science. 2012;335 (6074):1373-1376. [55] Su W, Hopkins S, Nesser NK, et al. The p53 transcription factor modulates microglia behavior through microRNA-dependent regulation of c-Maf. J Immunol. 2014;192(1):358-366. [56] McCauley LK. c-Maf and you won't see fat. J Clin Invest. 2010;120(10):3440-3442. [57] Tseng KY, Lin S. Zinc finger factor 521 enhances adipogenic differentiation of mouse multipotent cells and human bone marrowmesenchymal stem cells. Oncotarget. 2015;6(17):14874-14884. [58] Ahmadian M, Suh JM, Hah N, et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19(5):557-566. [59] Li R, Liang L, Dou Y, et al. Mechanical strain regulates osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells. Biomed Res Int. 2015;2015:873251.
|
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Li Cai, Zhao Ting, Tan Ge, Zheng Yulin, Zhang Ruonan, Wu Yan, Tang Junming. Platelet-derived growth factor-BB promotes proliferation, differentiation and migration of skeletal muscle myoblast [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1050-1055. |
| [3] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [4] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [5] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [6] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [7] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [8] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [9] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [10] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [11] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [12] | Song Kaikai, Zhang Kai, Jia Long. Microenvironment and repair methods of peripheral nervous system injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 651-656. |
| [13] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [14] | Zhou Wu, Wang Binping, Wang Yawen, Cheng Yanan, Huang Xieshan. Transforming growth factor beta combined with bone morphogenetic protein-2 induces the proliferation and differentiation of mouse MC3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3630-3635. |
| [15] | Zhu Rui, Zeng Qing, Huang Guozhi. Ferroptosis and stroke [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3734-3739. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||