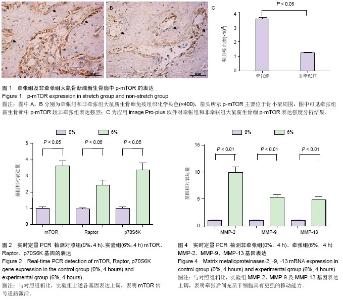
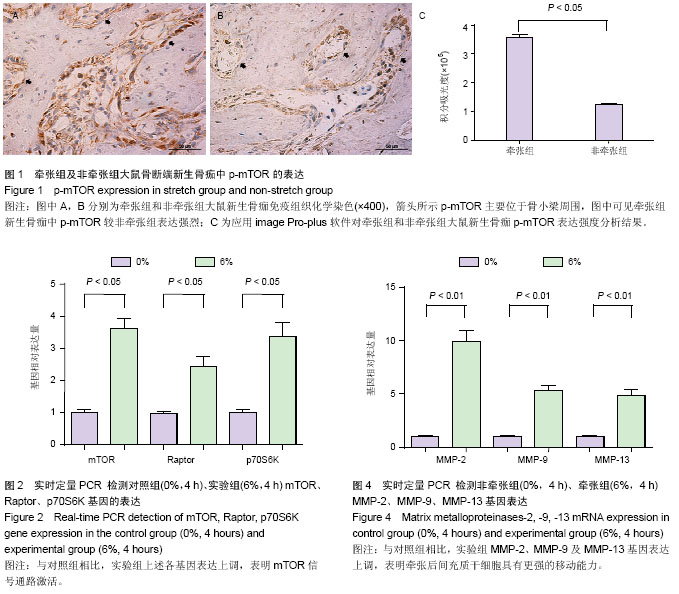
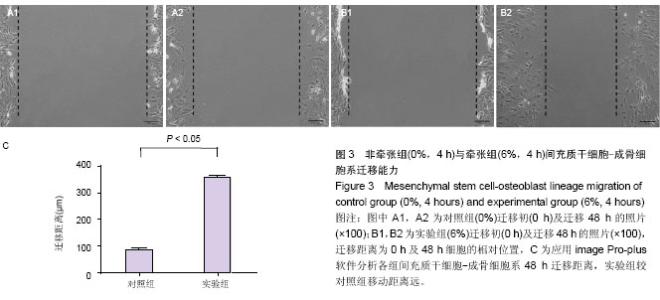
| [1] McCarthy JG, Schreiber J, Karp N, et al. Lengthening the human mandible by gradual distraction. Plast Reconstr Surg. 1992;89(1):1-8.
[2] Wang L, Cao J, Lei DL, et al. Application of nerve growth factor by gel increases formation of bone in mandibular distraction osteogenesis in rabbits. Br J Oral Maxillofac Surg. 201048(7):515-519.
[3] Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301-313.
[4] Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829-834.
[5] Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 2015;16(3):239-253.
[6] Du Z, Wang L, Zhao Y, et al. Sympathetic denervation- induced MSC mobilization in distraction osteogenesis associates with inhibition of MSC migration and osteogenesis by norepinephrine/adrb3. PLoS One. 2014;9(8):e105976.
[7] Chung KM, Hsu SC, Chu YR, et al. Fibroblast activation protein (FAP) is essential for the migration of bone marrow mesenchymal stem cells through RhoA activation. PLoS One. 2014;9(2):e88772.
[8] Wang X, Wang Y, Gou W, et al. Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int Orthop. 2013;37(12):2491-2498.
[9] Hara K, Maruki Y, Long X, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002; 110(2):177-189.
[10] Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14(14):1296-1302.
[11] Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274-293.
[12] Singha UK, Jiang Y, Yu S, et al. Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells. J Cell Biochem. 2008;103(2):434-446.
[13] Kim J, Jung Y, Sun H, et al. Erythropoietin mediated bone formation is regulated by mTOR signaling. J Cell Biochem. 2012;113(1):220-228.
[14] Hornberger TA, Stuppard R, Conley KE, et al. Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J. 2004;380(Pt 3):795-804.
[15] Nakai N, Kawano F, Oke Y, et al. Mechanical stretch activates signaling events for protein translation initiation and elongation in C2C12 myoblasts. Mol Cells. 2010;30(6):513-518.
[16] Wu MH, Lo JF, Kuo CH, et al. Endothelin-1 promotes MMP-13 production and migration in human chondrosarcoma cells through FAK/PI3K/Akt/mTOR pathways. J Cell Physiol. 2012;227(8):3016-3026.
[17] Zhang L, Wang H, Zhu J, et al. FTY720 reduces migration and invasion of human glioblastoma cell lines via inhibiting the PI3K/AKT/mTOR/p70S6K signaling pathway. Tumour Biol. 2014v;35(11):10707-10714.
[18] Mofid MM, Inoue N, Atabey A, et al. Callus stimulation in distraction osteogenesis. Plast Reconstr Surg. 2002;109(5): 1621-1629.
[19] Farberg AS, Sarhaddi D, Donneys A, et al. Deferoxamine enhances bone regeneration in mandibular distraction osteogenesis. Plast Reconstr Surg. 2014;133(3):666-671.
[20] Alkaisi A, Ismail AR, Mutum SS, et al. Transplantation of human dental pulp stem cells: enhance bone consolidation in mandibular distraction osteogenesis. J Oral Maxillofac Surg. 2013;71(10):1758.e1-13.
[21] Xian L, Wu X, Pang L, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18(7):1095-1101.
[22] Sun H, Kim JK, Mortensen R, et al. Osteoblast-targeted suppression of PPARγ increases osteogenesis through activation of mTOR signaling. Stem Cells. 2013;31(10):2183- 2192.
[23] Zheng N, Ding X, Jahan R. Low concentration of rapamycin inhibits hemangioma endothelial cell proliferation, migration, and vascular tumor formation in mice. Curr Ther Res Clin Exp. 2014;76:99-103.
[24] Chen Y, Zheng L, Liu J, et al. Shikonin inhibits prostate cancer cells metastasis by reducing matrix metalloproteinase-2/-9 expression via AKT/mTOR and ROS/ERK1/2 pathways. Int Immunopharmacol. 2014;21(2):447-455.
[25] Kimura A, Kabasawa Y, Tabata Y, et al. Gelatin hydrogel as a carrier of recombinant human fibroblast growth factor-2 during rat mandibular distraction. J Oral Maxillofac Surg. 2014; 72(10): 2015-2031.
[26] Deshpande SS, Monson LA, Cavaliere CM, et al. Distraction osteogenesis following low-dose hyperfractionated irradiation in the rat mandible. J Oral Maxillofac Surg. 2013;71(8): 1465-1470.
[27] Zhang YB, Wang L, Jia S, et al. Local injection of substance P increases bony formation during mandibular distraction osteogenesis in rats. Br J Oral Maxillofac Surg. 2014;52(8): 697-702.
[28] Cao J, Wang L, Du ZJ, et al. Recruitment of exogenous mesenchymal stem cells in mandibular distraction osteogenesis by the stromal cell-derived factor-1/chemokine receptor-4 pathway in rats. Br J Oral Maxillofac Surg. 2013; 51(8):937-941.
[29] Wang T, Cao J, Du ZJ, et al. Effects of sympathetic innervation loss on mandibular distraction osteogenesis. J Craniofac Surg. 2012;23(5):1524-1528.
[30] Kim IS, Song YM, Hwang SJ. Osteogenic responses of human mesenchymal stromal cells to static stretch. J Dent Res. 2010;89(10):1129-1134.
[31] Sen B, Xie Z, Case N, et al. mTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow-derived mesenchymal stem cells. J Bone Miner Res. 2014;29(1):78-89.
[32] Wu Y, Zhang X, Zhang P, et al. Intermittent traction stretch promotes the osteoblastic differentiation of bone mesenchymal stem cells by the ERK1/2-activated Cbfa1 pathway. Connect Tissue Res. 2012;53(6):451-459.
[33] Case N, Thomas J, Xie Z, et al. Mechanical input restrains PPARγ2 expression and action to preserve mesenchymal stem cell multipotentiality. Bone. 2013;52(1):454-464. |