Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (8): 2014-2022.doi: 10.12307/2026.079
Previous Articles Next Articles
Bionic functional coating improves the integration of titanium implants and skin tissue interface
Tan Jing1, Li Li2, Wang Liangliang1, Qin Xiangyu1
- 1School of Agriculture and Life Science, Shanxi Datong University, Datong 037009, Shanxi Province, China; 2Department of Laboratory Medicine, Datong Fifth People’s Hospital, Datong 037009, Shanxi Province, China
-
Received:2025-02-27Accepted:2025-05-06Online:2026-03-18Published:2025-07-17 -
Contact:Tan Jing, MD, Associate professor, School of Agriculture and Life Science, Shanxi Datong University, Datong 037009, Shanxi Province, China -
About author:Tan Jing, MD, Associate professor, School of Agriculture and Life Science, Shanxi Datong University, Datong 037009, Shanxi Province, China -
Supported by:National Natural Science Foundation of China, No. 82001972 (to TJ); Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi Province, No. 2021L374 (to TJ)
CLC Number:
Cite this article
Tan Jing, Li Li, Wang Liangliang, Qin Xiangyu. Bionic functional coating improves the integration of titanium implants and skin tissue interface[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2014-2022.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
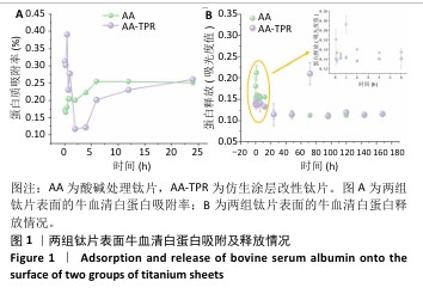
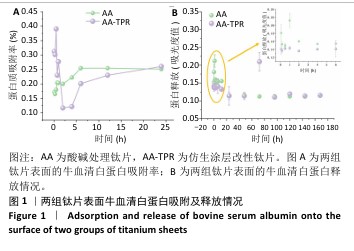
2.1 钛片表面的蛋白质吸附及释放情况 两组钛片表面蛋白质吸附率随时间变化,如图1A所示。仿生涂层改性钛片表面的蛋白质吸附变化表现为:0-60 min为蛋白质快速吸附和解吸附时期,此阶段吸附率变化波动较大,在45 min蛋白质吸附率达到最高值,60 min时蛋白质吸附率降至最低点;随后蛋白质吸附率开始稳步上升,此阶段为再吸附时期。60 min内仿生涂层改性钛片表面的蛋白质吸附率高于酸碱处理钛片,24 h时两组钛片表面的蛋白吸附率接近。 两组钛片表面的蛋白质释放情况,见图1B。仿生涂层改性钛片表面的蛋白质释放一直较为稳定,显示出少量稳定的释放情况;而酸碱处理钛片表面在前1 h内的蛋白质释放量较高。 综合蛋白质吸附与释放结果,仿生涂层改性钛片表面的蛋白质长期结合最稳定,与蛋白结合越稳定可能越有利于细胞在材料表面的增殖。 "
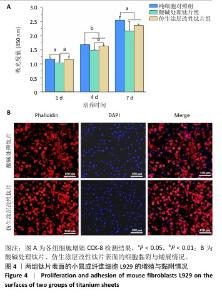
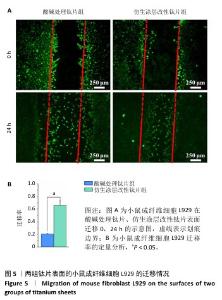
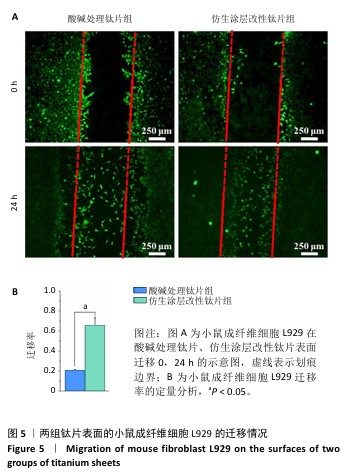
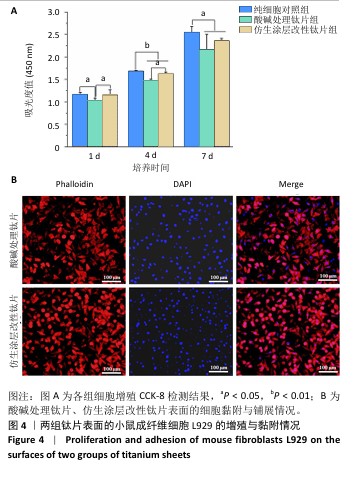
2.3 钛片表面的小鼠成纤维细胞L929的增殖黏附与迁移 培养1,4,7 d后,两组钛片表面的小鼠成纤维细胞L929增殖情况,见图4A所示。随着培养时间增加,3组细胞数量持续增加,培养1 d后,对照组与仿生涂层改性钛片组细胞数量多于酸碱处理钛片组(P < 0.05);培养4 d后,对照组与仿生涂层改性钛片组细胞数量多于酸碱处理钛片组(P < 0.05或P < 0.01),对照组细胞数量多于仿生涂层改性钛片组(P < 0.01);培养7 d后,对照组细胞数量多于酸碱处理钛片组、仿生涂层改性钛片组(P < 0.05)。培养4 d后的鬼笔环肽染色显示,小鼠成纤维细胞L929在两组钛片表面黏附并铺展,细胞分布较均匀,见图4B。 成纤维细胞迁移情况可以反映皮肤组织在材料表面的黏附迁移及整合情况。小鼠成纤维细胞L929在两组钛片表面的迁移能力检测结果,如图5所示。培养24 h后,对比酸碱处理钛片,仿生涂层改性钛片表面的小鼠成纤维细胞L929迁移更明显,原划痕边界的细胞大量向中间迁移,并且细胞呈整体迁移状态,表明仿生涂层改性钛片表面对成纤维细胞迁移有更显著的促进作用。"
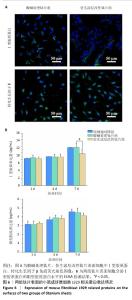
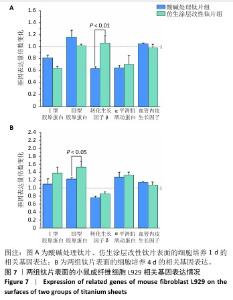
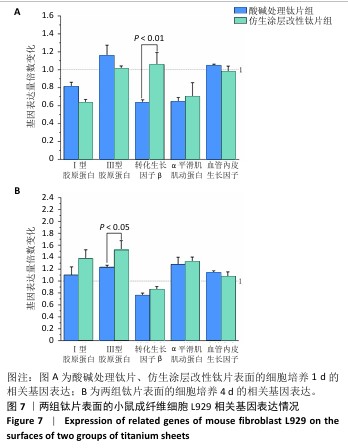
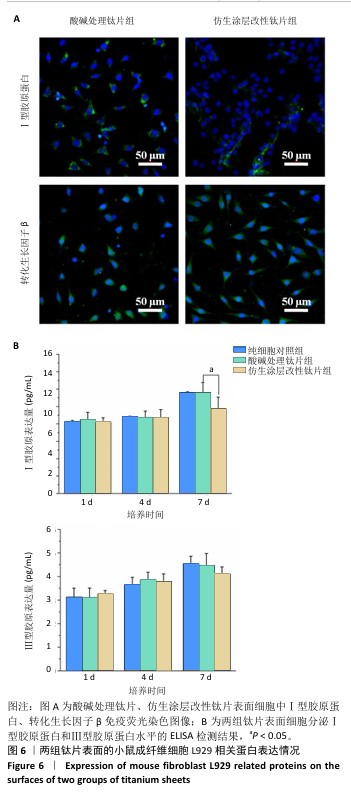
2.4 钛片表面的小鼠成纤维细胞L929的基质分泌、相关蛋白与因子表达 成纤维细胞的细胞基质分泌及相关蛋白表达情况对皮肤组织修复有着重要的作用。免疫荧光染色显示,两组钛片表面小鼠成纤维细胞L929均明显表达Ⅰ型胶原蛋白和转化生长因子β,见图6A。 ELISA检测结果显示,随着培养时间增加,两组钛片样表面小鼠成纤维细胞L929分泌的Ⅰ型和Ⅲ型胶原蛋白水平持续增加,酸碱处理钛片组培养7 d的Ⅰ型胶原蛋白水平高于仿生涂层改性钛片组(P < 0.05),两组间培养不同时间点的Ⅲ型胶原蛋白水平比较差异均无显著性意义(P > 0.05),见图6B。 利用qRT-PCR进一步检测两组钛片表面小鼠成纤维细胞L929中Ⅰ型胶原蛋白、Ⅲ型胶原蛋白、转化生长因子β、α-平滑肌肌动蛋白和血管内皮生长因子mRNA表达情况,结果如图7所示。Ⅰ型和Ⅲ型胶原蛋白反映细胞外基质沉积情况,转化生长因子β和α-平滑肌肌动蛋白是成纤维细胞活化与创面收缩关键因子,血管内皮生长因子为血管生成的关键调控因子,这些因子的表达情况可以反映材料对组织愈合的作用。仿生涂层改性钛片组培养1 d的转化生长因子β mRNA表达高于酸碱处理钛片组(P < 0.01),培养4 d的Ⅲ型胶原蛋白mRNA表达高于酸碱处理钛片组(P < 0.05)。成纤维细胞分泌的Ⅰ型和Ⅲ型胶原蛋白有利于新生细胞外基质的产生,对创面愈合起至关重要的作用,转化生长因子β可以使成纤维细胞部分分化为肌成纤维细胞,并表达α-平滑肌肌动蛋白,有利于伤口边缘的收缩。综合因子表达结果,仿生涂层改性钛片表面在促进成纤维细胞分泌Ⅲ型胶原蛋白和转化生长因子β方面表现出显著优势。"
| [1] SHRIVAS S, SAMAUR H, YADAV V, et al. Soft and Hard Tissue Integration around Percutaneous Bone-Anchored Titanium Prostheses: Toward Achieving Holistic Biointegration. ACS Biomater Sci Eng. 2024;10(4): 1966-1987. [2] ABDALLAH MN, BADRAN Z, CIOBANU O, et al. Strategies for Optimizing the Soft Tissue Seal around Osseointegrated Implants. Adv Healthc Mater. 2017;6(20).doi: 10.1002/adhm.201700549. [3] WEIGEL T, CHRIST B, DEMBSKI S, et al. Biomimetic Connection of Transcutaneous Implants with Skin. Adv Healthc Mater. 2023;12(30): e2301131. [4] SHAO J, KOLWIJCK E, JANSEN J A, et al. Animal models for percutaneous-device-related infections: a review. Int J Antimicrob Agents. 2017;49(6):659-667. [5] MURPHY NJ, GRAAN D, BALOGH ZJ. Percutaneous Titanium Elastic Nail Stabilization for Pelvic and Acetabular Fractures: Surgical Technique and Case Series. J Orthop Trauma. 2024;38(11):e371-e378. [6] ZHOU M, WANG J, WANG J, et al. Construction of a Localized and Long-Acting CCN2 Delivery System on Percutaneous Ti Implant Surfaces for Enhanced Soft-Tissue Integration. ACS Appl Mater Interfaces. 2023;15(19):22864-22875. [7] DING T, ZHANG L, HAN J, et al. Photo-Responded Antibacterial Therapy of Reinfection in Percutaneous Implants by Nanostructured Bio-Heterojunction. Small. 2023;19(7):e2206265. [8] WAN R, LI W, YANG K, et al. Immunomodulatory and bone regenerative properties of copper/procyanidins-modified titanium surfaces. Biomater Adv. 2025;169:214199-214199. [9] TAN J, LI L, LI B, et al. Titanium Surfaces Modified with Graphene Oxide/Gelatin Composite Coatings for Enhanced Antibacterial Properties and Biological Activities. ACS Omega. 2022;7(31):27359-27368. [10] DIAS LFG, COSTA RC, SACRAMENTO CM, et al. Tailoring bisphosphonate-doped titanium films to optimally couple cellular responses and antibacterial activity for biomedical applications. Biointerphases. 2024;19(3):031002. [11] WANG WR, LI J, GU JT, et al. Optimization of Lactoferrin-Derived Amyloid Coating for Enhancing Soft Tissue Seal and Antibacterial Activity of Titanium Implants. Adv Healthc Mater. 2023;12(11): e2203086. [12] 唐恺,王富,陈吉华.钛表面的仿生改性促进软组织黏附研究进展[J].中国实用口腔科杂志,2022,15(6):740-744. [13] NICOLÒ A, RENKO VD. Biomedical applications of solid-binding peptides and proteins. Materials Today Bio. 2023;19:100580. [14] WERONIKA J, JOANNA M, KAROLINA P, et al. Phage display and other peptide display technologies. FEMS Microbiol Rev. 2021;46(2):fuab052. [15] WU J, YANG M, HUANG Y, et al. Enhancing the Biological Performance of Titanium Alloy through In Situ Modulation of the Surface Nanostructure: Near-Infrared-Responsive Antibacterial Function and Osteoinductivity. ACS Appl Bio Mater. 2024;7(6):3900-3914. [16] 刘婉珍,周文昊,白天,等.钛基植入物表面抗菌涂层改性的研究进展[J].中国材料进展,2024,43(11):988-994. [17] 王泽华,程佳蕙,高啟坤,等.钛纳米管表面固定GL13K抗菌肽的实验研究[J].安徽医科大学学报,2019,54(2):251-255. [18] 谭婧.经皮钛植入体的纳米结构化表面改性及其生物学性能[D].成都:西南交通大学,2017. [19] SUN Y, TAN J, WU B, et al. Identification and binding mechanism of phage displayed peptides with specific affinity to acid–alkali treated titanium. Colloids Surf B Biointerfaces. 2016;146:307-317. [20] LI Y, FELLANDER-TSAI L. The bone anchored prostheses for amputees - Historical development, current status, and future aspects. Biomaterials. 2021;273:120836. [21] STICH T, ALAGBOSO F, KŘENEK T, et al. Implant-bone-interface: Reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng Transl Med. 2021;7(1):e10239. [22] ZHANG C, PAN Y, DUAN G, et al. A biomimetic calcium phosphate nanowire coating on titanium surface enhances osteoimmunomodulation and osteointegration. Composites Part B. 2024;280. doi:10.1016/j.compositesb.2024.111480 [23] 程新奇,邵龙辉,沈华侨,等.铜锶二元掺杂硅酸钙涂层改性钛合金的促成骨和抗菌效应[J].中国组织工程研究,2025,29(22): 4639-4646. [24] TOLLABI M, POURSALEHI Z, MEHRAFSHAR P, et al. Insight into the role of integrins and integrins-targeting biomaterials in bone regeneration. Connect Tissue Res. 2024;65(5):343-363. [25] LIU L, WANG J, LI Y, et al. Laminin 332-Functionalized Coating to Regulate the Behavior of Keratinocytes and Gingival Mesenchymal Stem Cells to Enhance Implant Soft Tissue Sealing. Regen Biomater. 2022;9:rbac054. [26] BODA SK, APARICIO C. Dual Keratinocyte-Attachment and Anti-Inflammatory Coatings for Soft Tissue Sealing around Transmucosal Oral Implants. Biomater Sci. 2022;10(3):665-677. [27] VILLEGAS M, BAYAT F, KRAMER T, et al. Emerging Strategies to Prevent Bacterial Infections on Titanium-Based Implants. Small. 2024;20(46): e2404351. [28] 严新安,WALTER M,林弋翔,等.临床细菌生物膜感染新型治疗方案研究进展[J].华西医学,2023,38(8):1276-1280. [29] MU S, ZHU Y, WANG Y, et al. Cationic Polysaccharide Conjugates as Antibiotic Adjuvants Resensitize Multidrug-Resistant Bacteria and Prevent Resistance. Adv Mater. 2022;34(41):e2204065. [30] KHAN SA, SHAKOOR A. Recent Strategies and Future Recommendations for the Fabrication of Antimicrobial, Antibiofilm, and Antibiofouling Biomaterials. Int J Nanomedicine. 2023;18:3377-3405. [31] LI Y, TAN J, LIU Z, et al. Antibacterial Activity and Cyto-/Tissue-Compatibility of Micro-/Nano-Structured Titanium Decorated with Silver Nanoparticles. J Biomed Nanotechnol. 2018;14(4):675-687. [32] MEININGER M, MEININGER S, GROLL J, et al. Silver and copper addition enhances the antimicrobial activity of calcium hydroxide coatings on titanium. J Mater Sci Mater Med. 2018;29(5):61. [33] SHARMA S, RAI VK, NARANG RK, et al. Collagen-based formulations for wound healing: A literature review. Life Sci. 2022;290:120096. [34] LICHTMAN MK, OTERO-VINAS M, FALANGA V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016;24(2):215-222. [35] LE LTT, GIANG NN, CHIEN PN, et al. Enhancement of Wound Healing Efficacy by Chitosan-based Hydrocolloid on Sprague Dawley Rats. In Vivo. 2023;37(3):1052-1064. |
| [1] | Fu Lyupeng, Yu Peng, Liang Guoyan, Chang Yunbing. Electroactive materials applied in spinal surgery [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2113-2123. |
| [2] | Peng Zhiwei, Chen Lei, Tong Lei. Luteolin promotes wound healing in diabetic mice: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1398-1406. |
| [3] | Yu Huifen, Mo Licun, Cheng Leping. The position and role of 5-hydroxytryptamine in the repair of tissue injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1196-1206. |
| [4] | Yan Qiquan, Yang Libin, Li Mengjun, Ni Yazhuo, Chen Keying, Xu Bo, Li Yaoyang, Ma Shiqing, Li Rui, Li Jianwen. Preparation and antibacterial properties of porcine small intestinal submucosal composite nanohydroxyapatite bioscaffold loaded with antimicrobial peptide KR-12-a5 [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 384-394. |
| [5] | Zhang Zhaowei, Chen Ouzile, Bai Mingru, Wang Chenglin. Therapeutic potential of bioactive substances secreted by dental mesenchymal stem cells for bone repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 163-174. |
| [6] | Luo Wenbin, Li Ruoyun, Pan Chaofan, Luo Changjiang. Engineered exosomes for repairing tissue damage: application potential, excellent biological stability, and targeting specificity [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 204-217. |
| [7] | Chang Jinxia, Liu Yufei, Niu Shaohui, Wang Chang, Cao Jianchun. Visualization analysis of macrophage polarization in tissue repair process [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1486-1496. |
| [8] | Li Jialin, Zhang Yaodong, Lou Yanru, Yu Yang, Yang Rui. Molecular mechanisms underlying role of mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1512-1522. |
| [9] | Zhang Yu, Xu Ruian, Fang Lei, Li Longfei, Liu Shuyan, Ding Lingxue, Wang Yuexi, Guo Ziyan, Tian Feng, Xue Jiajia. Gradient artificial bone repair scaffold regulates skeletal system tissue repair and regeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 846-855. |
| [10] | Xiong Zhenghua, Zhou Jianghong, Shen Yi, Han Xuesong . Mesenchymal stem cells and extracellular vesicles in repair of endometrial injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6782-6791. |
| [11] | Zhang Tong, Wang Yan, Yang Chunjia, Yue Qingkun, Wu Qingtian. Role of functional hydrogels in tissue repair after traumatic brain injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 6110-6117. |
| [12] | Deng Yunyi, Chen Shichao, Luo Mingdong, Li Ruotong, Lan Xiaorong, Yu Ke, Li Guangwen. Gold nanoparticle @ mesoporous silica modified titanium implants promote osteogenic differentiation under high glucose conditions [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(22): 4694-4701. |
| [13] | Li Qingyin, Li Linhua, Zhang Chunle, Fu Ping. Endothelial progenitor cell and mesenchymal stem cell therapy for vascular stent-associated diseases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 4091-4101. |
| [14] | Ye Chao, Liu Xiaohong. Regulatory strategies for foreign body reactions in biomaterials [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3513-3520. |
| [15] | Qin Jingjie, Guo Zige, Li Rui, Ma Shiqing, Lu Ruijie, Li Mengjun. Modification with bone forming peptide 1 and polydopamine coating to improve bioactivity of polyetheretherketone surface [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3318-3325. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||